Aurich Lawson
In 1972, Janet Rowley sat at her eating room desk and lower tiny chromosomes from pictures she had taken in her laboratory. One at a time, she snipped out the small figures her kids teasingly referred to as paper dolls. She then in moderation laid them out in 23 matching pairs—and warned her children to not sneeze.
The physician-scientist had simply mastered a brand new chromosome-staining methodology in a year-long sabbatical at Oxford. Nevertheless it used to be within the eating room of her Chicago house the place she made the invention that may dramatically regulate the process most cancers analysis.
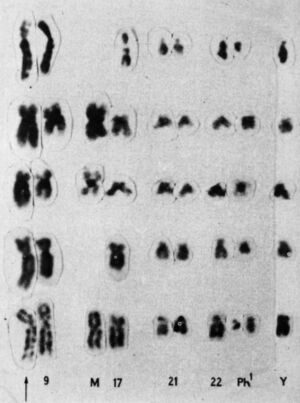 Amplify / Rowley’s 1973 partial karyotype appearing the 9;22 translocationLooking over the chromosomes of a affected person with acute myeloid leukemia (AML), she discovered that segments of chromosomes 8 and 21 had damaged off and swapped puts—a genetic business referred to as a translocation. She seemed on the chromosomes of different AML sufferers and noticed the similar transfer: the 8;21 translocation.
Amplify / Rowley’s 1973 partial karyotype appearing the 9;22 translocationLooking over the chromosomes of a affected person with acute myeloid leukemia (AML), she discovered that segments of chromosomes 8 and 21 had damaged off and swapped puts—a genetic business referred to as a translocation. She seemed on the chromosomes of different AML sufferers and noticed the similar transfer: the 8;21 translocation.
Later that very same yr, she noticed every other translocation, this time in sufferers with a distinct form of blood most cancers, referred to as continual myelogenous leukemia (CML). Sufferers with CML had been recognized to hold a puzzling abnormality in chromosome 22 that made it seem shorter than standard. The abnormality used to be referred to as the Philadelphia chromosome after its discovery through two researchers in Philadelphia in 1959. Nevertheless it wasn’t till Rowley pored over her meticulously set eating desk that it turned into transparent why chromosome 22 used to be shorter—a piece of it had damaged off and traded puts with a small segment of chromosome 9, a 9;22 translocation.
Rowley had the primary proof that genetic abnormalities had been the reason for most cancers. She printed her findings in 1973, with the CML translocation printed in a single-author find out about in Nature. Within the years that adopted, she strongly advocated for the concept that the abnormalities had been important for most cancers. However she used to be to begin with met with skepticism. On the time, many researchers regarded as chromosomal abnormalities to be a results of most cancers, now not the opposite direction round. Rowley’s findings had been rejected from the distinguished New England Magazine of Drugs. “I were given form of amused tolerance initially,” she stated sooner than her demise in 2013.
The beginning of centered remedies
However the proof fastened temporarily. In 1977, Rowley and two of her colleagues on the College of Chicago recognized every other chromosomal translocation—15;17—that reasons an extraordinary blood most cancers referred to as acute promyelocytic leukemia. By means of 1990, over 70 translocations were recognized in cancers.
The importance fastened temporarily as neatly. Following Rowley’s discovery of the 9;22 translocation in CML, researchers found out that the genetic switch creates a fusion of 2 genes. A part of the ABL gene typically discovered on chromosome 9 turns into connected to the BCR gene on chromosome 22, growing the cancer-driving BCR::ABL fusion gene on chromosome 22. This genetic merger codes for a signaling protein—a tyrosine kinase—this is completely caught in “energetic” mode. As such, it forever triggers signaling pathways that lead white blood cells to develop uncontrollably.
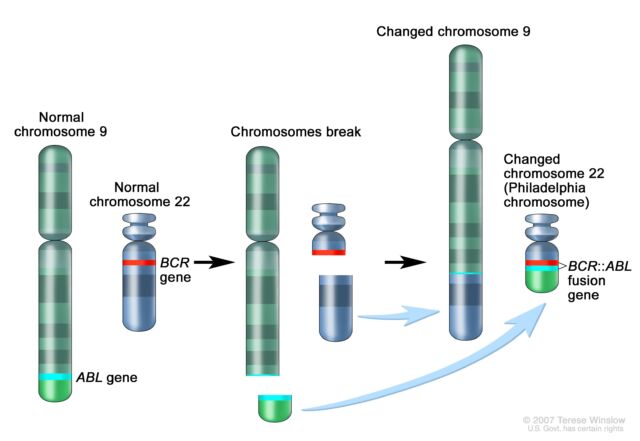 Amplify / Schematic of the 9;22 translocation and the introduction of the BCR::ABL fusion gene.
Amplify / Schematic of the 9;22 translocation and the introduction of the BCR::ABL fusion gene.
By means of the mid-Nineteen Nineties, researchers had evolved a drug that blocks the BCR-ABL protein, a tyrosine kinase inhibitor (TKI) referred to as imatinib. For sufferers within the continual segment of CML—about 90 p.c of CML sufferers—imatinib raised the 10-year survival charge from not up to 50 p.c to slightly over 80 p.c. Imatinib (bought as Gleevec or Glivec) earned approval from the Meals and Drug Management in 2001, marking the primary popularity of a most cancers remedy concentrating on a recognized genetic alteration.
With imatinib’s good fortune, centered most cancers treatments—aka precision drugs—took off. By means of the early 2000s, there used to be common passion amongst researchers to exactly establish the genetic underpinnings of most cancers. On the similar time, the progressive construction of next-generation genetic sequencing acted like jet gas for the hovering box. The generation eased the id of mutations and genetic abnormalities riding cancers. Sequencing is now regarded as same old care within the prognosis, remedy, and control of many cancers.
The advance of gene-targeting most cancers treatments skyrocketed. Categories of TKIs, like imatinib, expanded in particular speedy. There at the moment are over 50 FDA-approved TKIs concentrating on all kinds of cancers. For example, the TKIs lapatinib, neratinib, tucatinib, and pyrotinib goal human epidermal expansion issue receptor 2 (HER2), which runs amok in some breast and gastric cancers. The TKI ruxolitinib goals Janus kinase 2, which is continuously mutated within the uncommon blood most cancers myelofibrosis and the slow-growing blood most cancers polycythemia vera. CML sufferers, in the meantime, now have 5 TKI treatments to choose between.



.webp)










