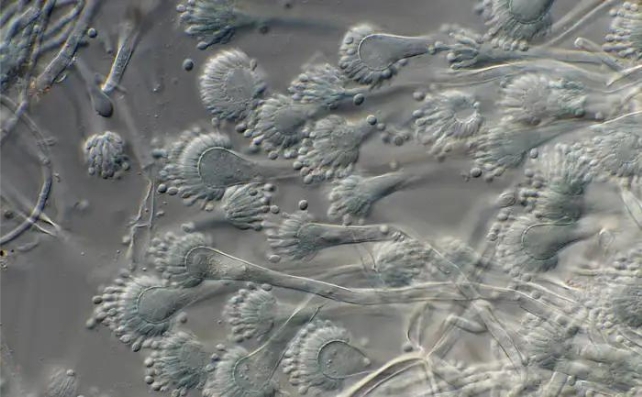
Individuals of the Andam lab traveled to Dartmouth-Hitchcock Scientific Middle to assemble micro organism remoted from blood samples from sufferers identified with bloodborne infections. They introduced the bacterial samples again to UAlbany for processing and genetic research. Credit score: Erin Frick
Antibiotics are a lifesaving device. But, because of their persistent overuse, microbes are evolving and growing immunity in opposition to them. Consequently, once-effective drugs can now not stave off infections, complicating remedy and lengthening mortality.
A College at Albany learn about not too long ago printed within the magazine Nature Communications recognized a brand new genetic mechanism that permits antimicrobial resistance to unfold amongst deadly micro organism.
The bacterium Klebsiella pneumoniae is the 3rd main explanation for blood infections globally. Usually discovered on human mucosal surfaces just like the respiration machine and gastrointestinal tract, when given a chance to invade, the micro organism could cause pneumonia and severe blood and urinary tract infections. Those infections can cause a formidable immune reaction that can result in organ failure and demise.
“We all know that many medically essential micro organism are now not responding to antibiotics, and a few are immune to more than one medication,” mentioned co-author Cheryl Andam, affiliate professor within the Division of Organic Sciences and the RNA Institute.
“On this learn about, with physicians on the Dartmouth-Hitchcock Scientific Middle, we sought to grasp the genetic elements that allow Klebsiella pneumoniae to increase antimicrobial resistance by means of examining the genome sequences of the micro organism from sufferers identified with bloodstream infections. This paintings offers a window into how those micro organism increase resistance genes and unfold them thru a inhabitants.”
The learn about represents an rising box known as genomic epidemiology, through which scientists monitor disease-causing micro organism throughout time and area the usage of entire genome sequencing to know the way the pathogen is evolving and spreading. This calls for figuring out the entire genes and genetic variants carried by means of person bacterial lines inside of a inhabitants.
The researchers analyzed the genetic sequences of 136 Okay. pneumoniae isolates gathered from grownup and pediatric sufferers with blood infections at Dartmouth-Hitchcock Scientific Middle over a five-year span (2017–2022). They recognized 94 distinct genetic sequences, indicating a excessive stage of genetic range inside the sampled Okay. pneumoniae inhabitants.
In addition they examined the genome sequences in opposition to 20 other antibiotics to decide whether or not the inhabitants incorporated lines recognized to be resistant. It did. The pattern incorporated 64 distinctive genes encoding resistance to 10 antimicrobial drug categories. Amongst those have been lines recognized to be hypervirulent and multidrug-resistant.

Genomic options of the 136 Okay. pneumoniae isolates from bloodstream an infection. Credit score: Nature Communications (2024). DOI: 10.1038/s41467-024-51374-x
Seriously, the staff found out how Okay. pneumoniae unfold resistance genes: by means of plasmids. Plasmids are cell genetic constructions that may elevate more than one resistance genes and unfold them to different micro organism. This mechanism facilitates the evolution of a more potent and extra resilient bacterial inhabitants.
“We discovered that plasmids are instrumental within the transmission of genes encoding enzymes that render many antibiotics useless,” mentioned Andam. “Maximum particularly, we discovered just about genetically equivalent plasmids, sporting genes encoding resistance to more than one antibiotics, in Okay. pneumoniae recovered from other sufferers separated by means of two years.
“Which means those plasmids can persist for a very long time and stay superb in disseminating and inflicting the emergence of multidrug resistant lines, which can be very tough to regard.”
This new figuring out will tell methods for public well being interventions geared toward controlling the unfold of high-risk bacterial clones.
“Persisted surveillance and additional genomic epidemiological research in well being care settings will deepen our figuring out of plasmid-facilitated antimicrobial resistance and the way this mechanism shapes well being dangers for susceptible sufferers and the broader neighborhood,” mentioned Andam.
“Antimicrobial resistance is an international danger as a result of microbial illnesses brought about by means of micro organism, viruses, parasites and fungi transform unresponsive to drug remedy and will motive life-threatening infections,” mentioned Prominent Professor Marlene Belfort, senior marketing consultant of the RNA Institute at UAlbany.
“That is an incredible downside as a result of medications that ordinarily kill those infectious organisms are changing into useless. It is idea that antimicrobial resistance is an identical danger to humankind as local weather trade and international starvation.
“What the Andam lab has proven is that genetic parts known as plasmids are what motive Klebsiella micro organism to transform to lines which are immune to a number of antibiotics. Those plasmids can transfer from one pathogenic microbe to any other, sporting with them genes that motive antibiotic resistance.
“Figuring out the mechanisms wherein antibiotic resistance spreads amongst Okay. pneumoniae is a essential step in opposition to figuring out the wider downside of antimicrobial resistance and growing therapies in opposition to unhealthy resistant lines.”
Additional information:
Odion O. Ikhimiukor et al, Clonal background and routes of plasmid transmission underlie antimicrobial resistance options of bloodstream Klebsiella pneumoniae, Nature Communications (2024). DOI: 10.1038/s41467-024-51374-x
Equipped by means of
College at Albany
Quotation:
Genome series research identifies new driving force of antimicrobial resistance (2024, September 20)
retrieved 21 September 2024
from
This report is matter to copyright. Except any honest dealing for the aim of personal learn about or analysis, no
section is also reproduced with out the written permission. The content material is supplied for info functions best.