In Switzerland, some 156,900 other folks have Alzheimer’s or any other type of dementia, and that is anticipated to upward thrust to 315,400 via 2050 in line with the organisation Alzheimer Schweiz.
Mtv / Courtesy Everett Assortment
Pay attention to the thing
Listening the thing
Toggle language selector
English (US)
English (British)
Generated with synthetic intelligence.
Swiss drugs regulator Swissmedic is predicted to come to a decision via the top of the yr whether or not to approve the primary new drug for Alzheimer’s illness in twenty years. The verdict received’t be simple.
This content material was once printed on
September 30, 2024 – 09:00
Jessica Davis Plüss

Jessica covers the nice, the unhealthy, and the unpleasant relating to giant world corporations and their have an effect on in Switzerland and in a foreign country. She’s at all times in search of a Swiss connection together with her local San Francisco and can luckily speak about why her place of birth has produced one of the most biggest inventions however can’t appear to unravel its housing disaster.
Extra from this creator
English Division
Alzheimer’s illness has baffled researchers for many years. Drugmakers have poured billions into the illness, which slowly destroys reminiscence and pondering abilities, however haven’t pop out with a brand new drug for no less than two decades.
This modified in July 2023 when america Meals and Drug Management authorized lecanemab, offered beneath the title Leqembi, for the remedy of early Alzheimer’s illness. Since then, it has additionally been authorized via government in Japan, China and South Korea. The drug is the primary to handle each the indications of reminiscence loss and what’s believed to be an underlying reason for the illness.
+ Get a very powerful information from Switzerland for your inbox
“Remaining yr was once a significant step ahead for Alzheimer’s analysis,” stated Andrea Pfeifer, founder and CEO of AC Immune, a Lausanne-based biotech company that has been operating on Alzheimer’s treatments for over two decades. “No new medication had come in the marketplace for goodbye that individuals stopped believing it was once even imaginable to regard the illness.”
This euphoria didn’t closing lengthy in Europe. In July 2024, the Eu Medications Company overview committee advisable rejecting the drug. They argued the dangers outweigh the advantages, mentioning protection issues equivalent to swelling and bleeding within the mind.
A month later, the United Kingdom regulatory company approved lecanemab however the Nationwide Institute for Well being and Care Excellence, which assesses medication’ cost-effectiveness, didn’t suggest it for repayment. It argued the fee, which is $26,500 (CHF22,300) a yr in america (and confidential in the United Kingdom) was once too prime relative to the advantages.
Extra
Extra
Drugmakers are in any case making medication for girls
This content material was once printed on
Sep 13, 2024
Intercourse and gender are hardly regarded as in drug construction. There’s now a motion, together with in Switzerland, to modify this.
Learn extra: Drugmakers are in any case making medication for girls
Alzheimer’s sufferers in Switzerland are actually eagerly anticipating a choice via the Swiss regulator, Swissmedic, which is predicted via the top of 2024. The verdict is a ways from simple within the face of such a lot of diverging reviews. The regulator has to weigh the advantages and dangers of a drug for a life-threating illness that also isn’t absolutely understood and hasn’t observed a step forward in many years.
The verdict received’t simply impact hundreds of other folks in danger or residing with Alzheimer’s in Switzerland however may even ship a sign to drugmakers about how a lot to put money into new remedies for the illness.
About Alzheimer’s Illness.
Alzheimer’s illness is the main reason for dementia on the earth. Dementia is an overarching time period that refers to a variety of signs affecting cognitive talents, whilst Alzheimer’s illness is a particular form of dementia characterized via revolutionary reminiscence loss and cognitive decline.
Globally, over 55 million other folks be afflicted by dementia, as much as 70% have Alzheimer’s illness. In Switzerland, some 156,900 other folks have Alzheimer’s or any other type of dementia, and that is anticipated to upward thrust to 315,400 via 2050 in line with the organisation Alzheimer Suisse.
The illness slowly destroys reminiscence and pondering abilities, and ultimately, the facility to hold out easy duties. The Global Well being Group estimates that the illness prices healthcare programs round $1.3 trillion annually.
Many unknowns
The diverging perspectives on lecanemab are an indication of the way arduous it’s been to make development at the illness. There’s no conclusive proof of what if truth be told reasons the illness. As of now, there’s nonetheless no authorized blood check to stumble on whether or not anyone has Alzheimer’s and what sort of it has stepped forward.
Medication so far have most effective been ready to ease signs of the illness equivalent to reminiscence loss. However catching the illness at that time is just too past due as a result of reminiscence loss can’t be reversed.
“You want to regard the illness early, that means earlier than the mind is broken,” stated Pfeifer. To take action “we want to to find out if an individual is liable to creating Alzheimer’s 15 to two decades earlier than signs get started.”
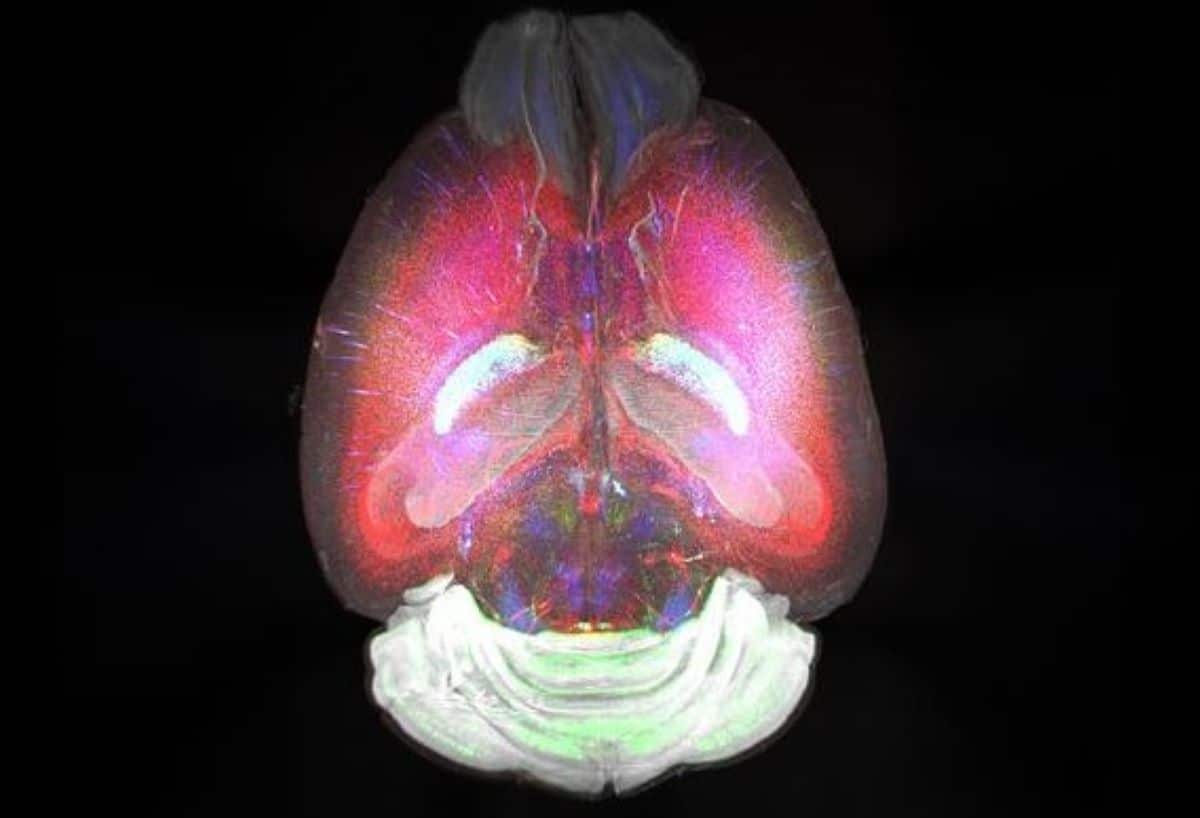
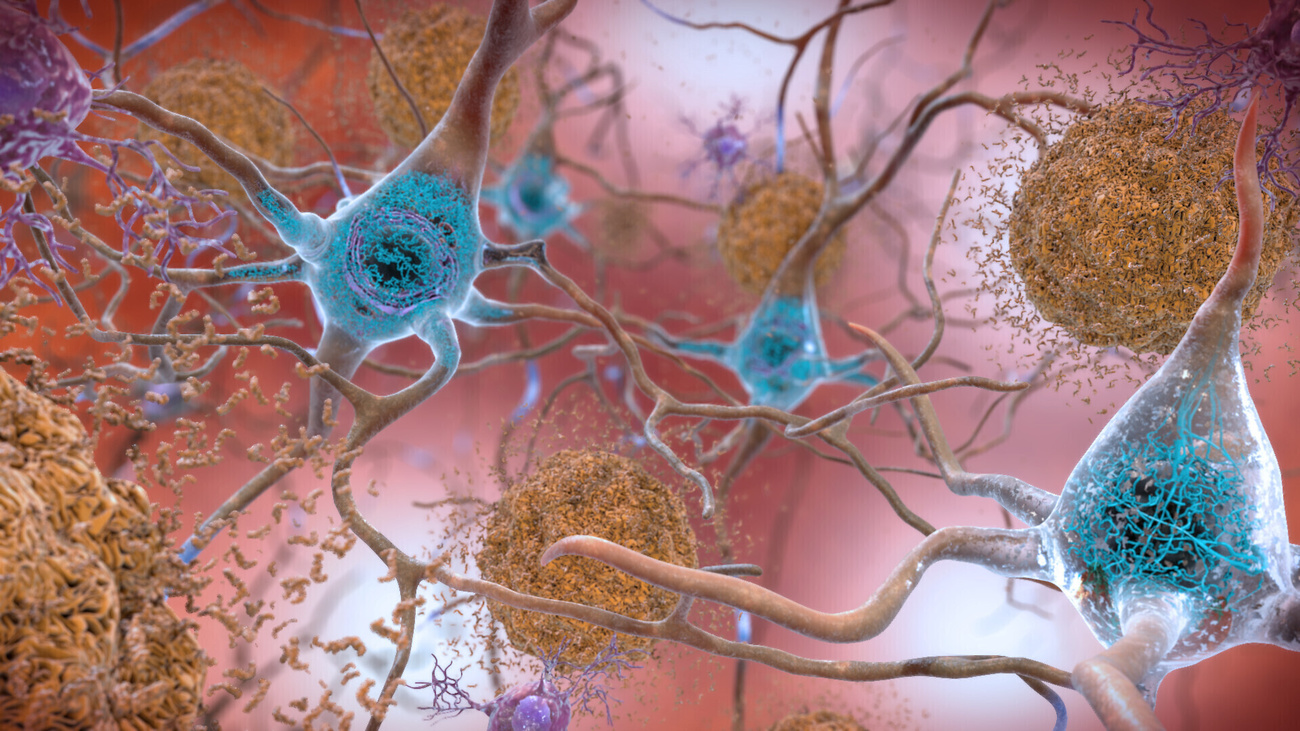
This has led drugmakers to 0 in on what occurs within the mind of Alzheimer’s sufferers. Mind scans display atypical ranges of amyloid beta protein, which accumulates to shape plaques within the mind that disrupt mobile serve as. Lecanemab is a part of a brand new staff of substances that concentrate on those plaques.
Extra
Extra
Step forward Alzheimer’s drug produced in Switzerland
This content material was once printed on
Jul 7, 2023
Biogen’s new manufacturing facility in Switzerland is the one manufacturer of the lively element in Leqembi, authorized to regard Alzheimer’s illness.
Learn extra: Step forward Alzheimer’s drug produced in Switzerland
However measuring the volume of plaque within the mind isn’t sufficient to mention whether or not the medication stave off reminiscence loss. Some individuals who have plaques by no means pass directly to broaden dementia. Some medication decreased plaques however didn’t result in any adjustments in reminiscence loss or cognition.
Lecanemab, which is offered via US company Biogen and Jap company Eisai, was once the primary drug that now not most effective decreased plaques within the mind but in addition slowed symptom development. The most important trialExternal hyperlink of the drug with over 1,700 other folks with early Alzheimer’s confirmed the drug slowed cognitive decline via 27% in comparison to a placebo in 18 months.
Whilst researchers celebrated this as a watershed second for the illness, what it way for sufferers has been tricky for regulators to interpret. In accordance to a couple mavens, this would stay the dementia at bay for a trifling 5 months. The modest results would possibly now not also be noticeable to a affected person or physician, say some expertsExternal hyperlink.
“At an early degree of the illness, the lively element reduces the damaging protein deposits within the mind and thus delays the development of the illness,” wrote Jacqueline Wettstein, a spokesperson for the Alzheimer Suisse affiliation, via e-mail. “Alternatively, lecanemab can neither remedy nor prevent Alzheimer’s illness.”
This advantage additionally needs to be weighed towards the unwanted effects together with mind swelling or microbleeds that can result in minor complications and in some circumstances, loss of life in line with the trial.
When the FDA authorized lecanemab, it stated the drug was once protected and confirmed clinically significant advantage. The Eu Medications Company got here to another conclusion, arguing the “advantages of remedy don’t seem to be sufficiently big to outweigh the dangers related to Leqembi (lecanemab)”.
Even if regulators have authorized the drug, some well being insurers, as in the United Kingdom, have refused to pay for it, arguing it prices an excessive amount of for the few advantages it supplies. The drug is priced at $26,500 a yr in america however this doesn’t come with the prices of the bi-weekly infusions and follow-up.
Rewarding breakthroughs
Antonella Santuccione Chadha, a neuroscientist who labored on Alzheimer’s drug construction and now leads the Zurich-based Girls’s Mind Basis, says the benefit-risk equation needs to be considered within the wider context of Alzheimer’s analysis.
“I remember the fact that the dangers related to those medication are prime relative to the advantages,” stated Chadha. “However this can be the fee we need to pay to transport analysis ahead in this devastating illness that has no remedy.”
Within the closing decade greater than 200 analysis programmes had been both deserted or have failed in late-stage medical trials, when medication are examined in huge selection of other folks, in line with US-based well being researchExternal hyperlink company IQVIA
IQVIA estimates the full prices to broaden an Alzheimer’s drug is ready $5.6 billion when put next with $793.6 million for a most cancers drug.
Pfeifer says that US approval of lecanemab despatched a message to corporations like hers that it’s definitely worth the funding. The drug is estimated to generateExternal hyperlink $361 million globally in 2024.
“Those new medication is probably not easiest treatments, however they’re slowing cognitive decline in lots of sufferers,” stated Pfeifer. “If medication which are no less than moderately efficient aren’t authorized, who will put money into Alzheimer’s analysis to convey the following era of substances to the marketplace?”
A yr after lecanemab was once authorized, the FDA gave the fairway mild to a 2nd drug, donanemab, offered as Kisunla via US company Eli Lilly. The United Kingdom, Eu and Australian regulators are nonetheless assessing the drug.
Extra

Extra
Swiss analysis performed key function in new Alzheimer’s drug
This content material was once printed on
Jun 8, 2021
A newly authorized remedy for Alzheimer’s was once constructed at the foundations of Swiss clinical analysis, says the College of Zurich.
Learn extra: Swiss analysis performed key function in new Alzheimer’s drug
Some 160 clinicals trials are registered in america platform clinicaltrials.gov assessing 127 medication for Alzheimer’s illness. Analysis is now underway into blood diagnostic checks and new medication that take on irritation and different proteins past amyloid beta at the back of the illness.
AC Immune, the Lausanne-based biotech, has been operating for two decades on diagnostic checks and immunotherapies, that faucet the immune cells’ talent to transparent plaques from the mind. It now has 5 medication in medical trials, and could also be investigating new underlying reasons of the illness.
“Each and every learn about improves our figuring out of the illness. According to those successes, the following era will arrive even sooner and supply better advantages and stepped forward protection,” stated Pfeifer.
In Might 2024, the Jap company Takeda and AC Immune sealed a deal value $100 million up entrance and probably billions extra later if the corporate is a success. Beneath the deal, Takeda has an unique method to license world rights to one in every of its immunotherapies in medical trials.
Time for a remedy
It’s unclear how Swissmedic will rule on lecanemab. Eisai submitted an authorisation utility for the drug to Swissmedic in Might 2023. Based on SWI, a Swissmedic spokesperson stated it will probably’t proportion information about a pending determination. It isn’t atypical for the regulator to take greater than a yr to achieve a choice.
Extra
Extra
How drug costs are negotiated in Switzerland and past
This content material was once printed on
Apr 23, 2024
Switzerland’s pharmaceutical sector provides medication international, however now not all nations obtain them with the similar price ticket. Right here’s why.
Learn extra: How drug costs are negotiated in Switzerland and past
As Switzerland isn’t a part of the Eu Union, Swissmedic makes selections independently from the Eu Medications Company. They paintings with mavens to test that the product “meets the necessities for efficacy, high quality and protection”.
Remaining yr Swissmedic approved about 84% (41 new medication) of recent drug programs. America FDAExternal hyperlink authorized the similar proportion closing yr – 84% (55 new medication).
Despite the fact that lecanemab gives minimum advantage, Alzheimer’s sufferers in Switzerland are hoping for a good determination. Presently, other folks in Switzerland can most effective import the drug at their very own expense.
After years of analysis, we in any case have a drug in the house stretch that may no less than extend the illness at an early degree, stated Wettstein. Lecanemab can’t prevent Alzheimer’s illness. “But when it’s given at early degree of the illness, it may give other folks with the illness extra time.”
Edited via Virginie Mangin/ds
Articles on this tale