Proposals for the brand new definition of the tetrel bond shall be to be had for group evaluation in 2025, consistent with the World Union of Natural and Carried out Chemistry’s (Iupac) committee chair Giuseppe Resnati. The announcement follows the discharge of the pnictogen bond suggestions previous this 12 months as a part of a 20-year undertaking to officially explain the terminology round secondary bonding interactions after many years of bewilderment and misuse.
As a essentially non-visual self-discipline, chemistry wishes transparent and well-defined nomenclature to offer a competent and significant means for researchers to put across their findings to others. Whilst the hydrogen bond is universally recognised via the chemistry group, its extra difficult to understand kin – the halogen, pnictogen, chalcogen and tetrel bonds – are steadily lost sight of, both misnamed or misclassified as different sorts of interplay. In fresh many years, this in style misuse of terminology created a disjointed and inconsistent base of information throughout the literature which, in 2004, Iupac determined to take on head-on.
As the pro organisation liable for standardisation in chemistry, Iupac brings in combination researchers from around the chemical sciences to create a common clinical language. All suggestions cling a prison standing, with comments and consensus from the broader group taking part in a very important position in each new mission. However as chemistry evolves, so should its terminology, that means an enormous a part of the organisation’s position is to problem present definitions and conventions which not replicate a contemporary working out of chemistry.
Naming issues
Secondary bonding interactions are a chief instance of this very factor. Those susceptible, non-covalent interactions underpin analysis spaces as various as catalysis, supramolecular chemistry and organic chemistry, with hydrogen bonds probably the most broadly recognised instance.
For over 60 years, it used to be believed that hydrogen used to be the one component in a position to forming those bonds and the phrases ‘secondary bonding interplay’, ‘non-covalent bond’ and ‘hydrogen bond’ have been steadily used slightly interchangeably. Analysis within the 70s later known halogen atoms taking part in an identical interactions and next research demonstrated that different p-block components may additionally shape analogous bonds.
This larger nuance, coupled with the loss of particular names and definitions for those newly came upon interactions created a point of bewilderment within the box. ‘Folks have been the usage of this terminology wrongly within the literature or proposing new names and definitions. Other communities have been the usage of other phrases to mention precisely the similar factor,’ says Pierangelo Metrangolo, a supramolecular chemist and Iupac officer. ‘We realised there used to be a wish to distinguish every of those interactions obviously.’
Metrangolo, as a part of an Iupac committee, has spent the ultimate two decades running on tasks to increase a sequence of definitions and supporting documentation to lend a hand researchers extra successfully establish and classify those secondary bonding interactions. Every advice contains an evidence of the important thing options of the bond, adopted via an inventory of function experimental and theoretical proof for the interplay.
‘Folks wish to have authoritative paperwork they may be able to discuss with the place there’s a transparent definition, but additionally an extended checklist of notes the place you’ll get the parameters. If lots of the prerequisites are met, you recognize you’ve encountered that roughly interplay,’ says Metrangolo.
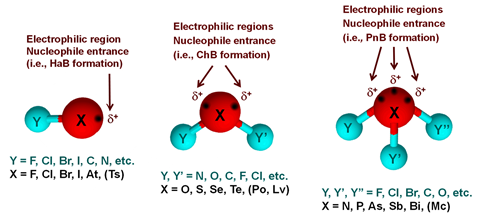
Previous this 12 months, the crew launched a definition for the pnictogen bond – the crowd 15 analogue of the hydrogen bond – with a last advice for staff 14’s tetrel bond anticipated in numerous years’ time. Crucially, this definition, as with others within the sequence, emphasises the elemental nature of the interplay. The named donor atom (the pnictogen) acts because the electrophile, making a predominantly electrostatic enchantment in opposition to a nucleophilic area of the acceptor. ‘The purpose used to be to fret that, because of an anisotropic distribution of the electron density attributable to the covalent bonds shaped via the pnictogen atom to different atoms within the molecule, one of the outer floor will also be electrophilic, even for components recurrently perceived as Lewis bases,’ explains Resnati.
By way of explicitly naming interactions consistent with the electrophilic contributor, the brand new phrases permit a transparent difference between an identical bonds, which in flip provides researchers a very important software to steer the ones interactions. ‘If we will be able to distinguish between a hydrogen bond and a pnictogen bond, we will be able to consider the criteria contributing to the pressure there,’ explains Steve Scheiner, any other committee member, specialising in computational chemistry. ‘However many of us don’t simply settle for the concept two electronegative atoms can if truth be told shape a phenomenal interplay.’
This lack of knowledge is an ongoing factor, with many researchers misclassifying their intermolecular interactions as extra acquainted hydrogen bonds. Then again, it used to be the crew’s unique paintings on redefining the hydrogen bond again in 2011 that first published the level of this downside and started to take the primary steps to deal with this systemic miscommunication.
Advanced working out
The hydrogen bond used to be first known over 100 years in the past and used to be at first explained as ‘a susceptible electrostatic chemical bond which paperwork between covalently-bonded hydrogen atoms and a strongly electronegative atom with a lone pair of electrons’ – which in observe supposed nitrogen, oxygen or fluorine.
Then again, over the second one part of the 20 th century, a rising frame of proof advised that possibly this slender definition didn’t inform the entire tale. ‘It used to be regarded as early on {that a} CH staff used to be incapable of forming a hydrogen bond. However within the 50s, some crystal constructions have been revealed which appeared like that they had CH–O hydrogen bonds,’ says Scheiner. ‘This used to be briefly poo-pooed via different crystallographers who argued it doesn’t matter what it appeared like, it couldn’t be a hydrogen bond as it used to be a CH.’
The spectroscopic standards for figuring out a hydrogen bond have been also referred to as into query. An early function signal used to be purple moving within the IR spectrum – the covalent bond involving the shared hydrogen produced a broader top shifted in opposition to a decrease frequency. Then again, via 1998, conclusive instances started to emerge the place this bond’s frequency used to be if truth be told shifted within the different route (referred to as a blue shift), triggering additional war of words as as to if those interactions might be referred to as a hydrogen bond.
In 2004, Iupac shaped a committee to guage and refine the prevailing definition of the hydrogen bond to replicate this modern working out and the crew have been cautious to keep away from the restrictions of the previous definition. ‘When you must describe a phenomenon you’ll focal point on many key options – the character of the interplay, the geometric options and so forth – however what characterises these kind of approaches is that you’re making an emphasis of 1 characteristic over the others,’ says Resnati who joined the crew in 2010. ‘The verdict made once we began this used to be no longer to concentrate on the working out of the interplay which would possibly evolve with time, no longer on anyone characteristic of the interplay, however at the very essence.’
After seven years, having evaluated an immense frame of literature documenting this evolving working out of the hydrogen bond, the crew proposed a brand new definition along an inventory of standards and traits to make stronger researchers in optimistically figuring out this kind of bond.
‘The hydrogen bond is a phenomenal interplay between a hydrogen atom from a molecule or a molecular fragment X–H by which X is extra electronegative than H, and an atom or a gaggle of atoms in the similar or a distinct molecule, in which there’s proof of bond formation.’
However, regardless of the intensive supporting clarification, the brand new definition first of all proved debatable. Ingrained reviews in regards to the nature of hydrogen bonding from around the group made attaining a consensus in particular difficult and in the end the paper went to over 20 referees earlier than receiving ultimate approval in 2011.
Increasing around the periodic desk
It used to be all the way through the process this collaborative and cross-disciplinary dialogue that the crew known the opposite key downside with Iupac’s present definition framework – lacking terminology. The loss of readability round those extra not too long ago came upon interactions left researchers to suggest their very own definitions for those intermolecular bonds, inflicting confusion and inconsistency right through the literature.
The time period ‘halogen bond’, particularly, used to be broadly misused and in 2010 the halogen bond definition mission used to be proposed, with the analogous staff 14–16 mission initiated in a while after. At a basic stage, every of those interactions is a part of the similar wider phenomenon: the asymmetric distribution of electron density can create a localised electropositive area, even on an electronegative atom. The ensuing electrostatic enchantment to a close-by nucleophilic area then creates a (most often) susceptible bond whose identification is made up our minds via the electrophilic atom.
Whilst the converting valency around the periodic desk leads to a few variations, the underlying idea at the back of hydrogen, halogen, chalcogen and pnictogen bonds is identical. Resnati’s crew have been prepared to verify consistency throughout this sequence of definitions, taking the semantic construction of the brand new hydrogen bond definition as a style for the remainder. ‘The extension of the mindset followed within the new definition of the hydrogen bond to different components used to be slightly simple and attaining a consensus for the halogen and the chalcogen bonds used to be certainly a lot more straightforward,’ he says.
The new pnictogen bond definition proved slightly tougher on this regard because the larger valency and extra numerous nature of the crowd’s components sophisticated the definition procedure. ‘In staff 15 you progress from components which might be most often giving covalent bonds – nitrogen – to components which might be metals – bismuth – with the intention to arrive at a consensus with other people with non-minor variations in the idea that in their use, bonding and interactions used to be truly difficult,’ Resnati explains. The mission addressed those problems with the overall definition:
‘[A] susceptible sexy interplay between an electrophilic area on a pnictogen atom in a molecular entity (in which the pnictogen is fascinated with different more potent bonds) and a nucleophilic area in any other, or the similar, molecular entity.’
This extra complexity supposed the crew spent a complete of six years running at the pnictogen bond definition, virtually double the time spent at the halogen and chalcogen bonds. Then again, this effort has no longer long gone unappreciated via the analysis group.
Readability eventually
Statistics compiled via American Chemical Society (ACS) publications show the level to which the chemical group has embraced this new terminology. Following the discharge of the definition in 2013, halogen bonds have been discussed a mean of 73 instances a 12 months throughout all ACS papers, in comparison with a mean of simply 9 instances a 12 months within the two decades previous the mission’s announcement. A an identical development may be rising for 2019’s chalcogen bond, with a mean of 9 instances extra annual mentions for the reason that advice used to be revealed.
Transparent and particular terminology additionally streamlines the method of analysis itself, offering citable phrases for researchers to make use of in publications. ‘It’s simplified my lifestyles,’ says Anthony Cozzolino, a supramolecular chemist at Texas Tech College. ‘The definitions lend a hand unify the sector and provides searchable names which, in flip, is helping me establish the analysis that I wish to be having a look via and goal my efforts.’ On the identical time, the superiority of secondary bonding interactions throughout such a lot of other portions of chemistry brings researchers into touch with different analysis spaces which will then information new methods drawn from each fields in combination.
However extra broadly, the eye surrounding those definitions has shone a gentle on those interactions as crucial and rising house of analysis. ‘Having the Iupac definitions lends credence to the sector that makes it more straightforward to push in new instructions,’ says Cozzolino. ‘In the case of investment, you’ll display there’s precedent for this chemistry which supplies weight to grant packages.’
An enormous a part of the good fortune of those definitions has been the emphasis put on consensus – a demand for all Iupac definitions – and the ACS’s Committee on Nomenclature, Terminology, and Symbols (NTS) is simply probably the most skilled our bodies invited to supply comments at the early proposals. ‘Definitions lend a hand us all talk the similar language and align round constant meanings,’ says Clay Harris, strategic projects chief for the ACS’s NTS committee. ‘We worth alternatives like this to lend a hand make certain that proposals paintings for chemistry practitioners and our world group of individuals. Those sure interactions make stronger the evolution of commonplace terminology used within the world observe of chemistry.’
Resnati’s crew are actually running at the ultimate definition within the sequence however the committee be expecting arriving at a consensus for staff 14’s tetrel bond will turn out probably the most difficult but. The central significance of carbon in such a lot of spaces of chemistry, and the distinction with the wildly other houses and behaviours of heavier staff individuals equivalent to lead will most likely make the main points of the overall definition tough to determine and it might be years earlier than the overall definition is revealed. ‘To reach at a commonplace opinion shared via chemists with such other backgrounds shall be actually difficult however I believe that the former definitions – hydrogen, halogen, chalcogen, pnictogen – are proving that the way is significant,’ says Resnati.
Thank you additionally to Gautam Desiraju, Anthony Legon and Antonio Frontera for his or her contributions.