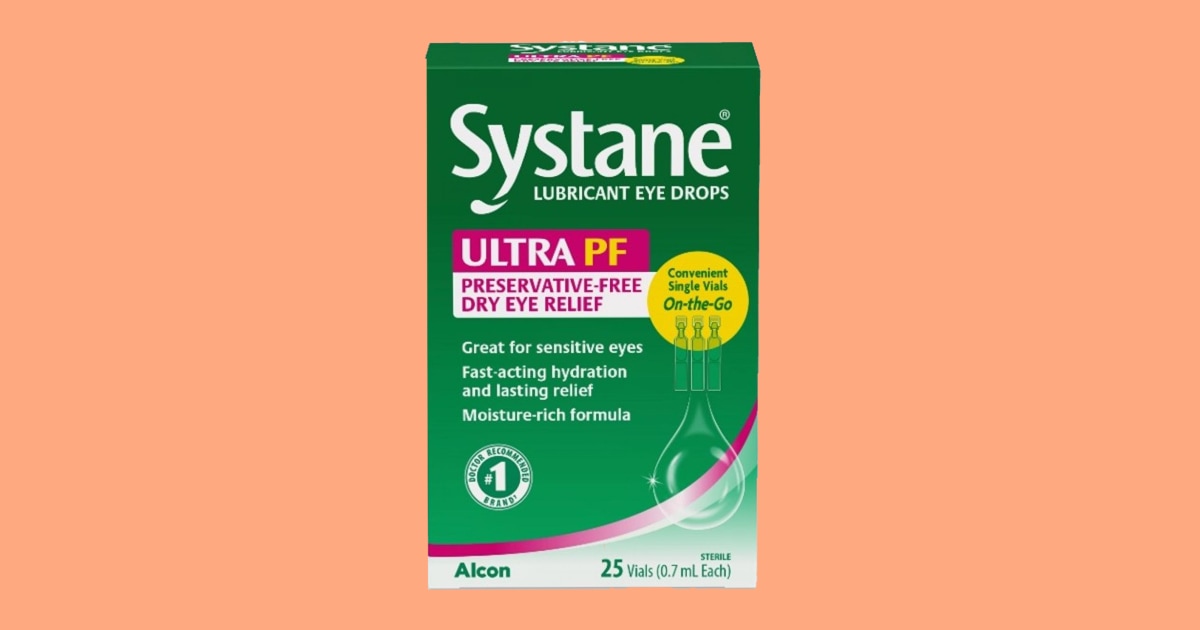
Eyedrops disbursed national are being recalled after a “fungal subject matter,” used to be discovered inside of an unopened vial of eyedrops. Alcon Laboratories Inc. issued a voluntary recall of 1 lot of 25-count Systane Lubricant Eye Drops Extremely PF Unmarried Vials On-the-Pass previous this week. A buyer notified the corporate of a overseas subject matter in a sealed vial which used to be later decided fungus. If fungus is presented to the eyes it will possibly motive eye infections that can be imaginative and prescient threatening, in line with the Meals and Drug Management.  Eyedrops recall.FDAIn uncommon instances, a watch an infection will also be life-threatening to immunocompromised other people. The FDA advises consumers who’ve the affected merchandise to prevent the use of them straight away and go back them to where of acquire for a reimbursement or substitute. Alcon has no longer gained any harm studies associated with this recall as of Dec. 24. The affected eyedrops are restricted to lot 10101, with an expiration date of September 2025. The product bundle is inexperienced and red with logo names “Systane,” and “ULTRA PF,” at the entrance of the bundle and “25 vials,” indexed because the bundle dimension. Those merchandise have been disbursed national to retail and web shops. Shoppers are recommended to file any hostile reactions associated with this recall on-line. Extra Trade Information
Eyedrops recall.FDAIn uncommon instances, a watch an infection will also be life-threatening to immunocompromised other people. The FDA advises consumers who’ve the affected merchandise to prevent the use of them straight away and go back them to where of acquire for a reimbursement or substitute. Alcon has no longer gained any harm studies associated with this recall as of Dec. 24. The affected eyedrops are restricted to lot 10101, with an expiration date of September 2025. The product bundle is inexperienced and red with logo names “Systane,” and “ULTRA PF,” at the entrance of the bundle and “25 vials,” indexed because the bundle dimension. Those merchandise have been disbursed national to retail and web shops. Shoppers are recommended to file any hostile reactions associated with this recall on-line. Extra Trade Information
Eyedrops recalled because of ‘vision-threatening’ fungus: Go back ASAP