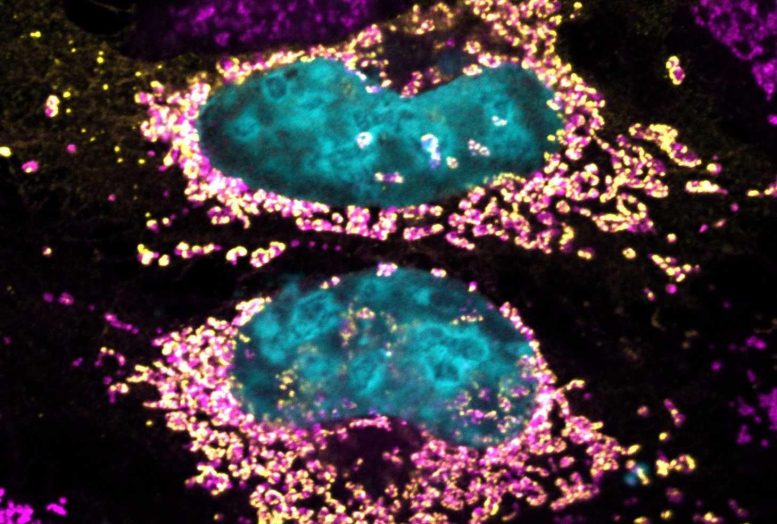
 This discovery by researchers suggests potential new treatments for autoimmune diseases and cancer, as Coxiella burnetii, a Gram-negative intracellular bacterium, was found to produce a previously unidentified protein with antioxidant properties.Recently, scientists at the University of São Paulo, in collaboration with Australian colleagues, uncovered a unique bacterial protein with the ability to maintain human cell health even when the cells are heavily burdened by bacteria. This breakthrough has the potential to lead to the development of new treatments for various diseases related to mitochondrial dysfunction, including cancer and autoimmune disorders. Mitochondria, known as the cell’s powerhouses, are essential for producing the energy required for cellular biochemical reactions.The research article is published in the journal PNAS. The team scrutinized over 130 proteins released by Coxiella burnetii upon invading host cells and identified at least one protein capable of extending cell longevity by directly influencing mitochondria.Upon invading host cells, C. burnetii releases a previously unknown protein, named mitochondrial coxiella effector F (MceF) by the authors. MceF interacts with glutathione peroxidase 4 (GPX4), an antioxidant enzyme located in the mitochondria, to enhance mitochondrial function by promoting an antioxidizing effect, preventing cell damage and death that may occur when pathogens replicate inside mammalian cells.“C. burnetii employs various strategies to prevent the death of invaded cells and multiply inside them. One such strategy is the modulation of GPX4 by MceF, a mechanism that we discovered and reported in this article. The relocation of these proteins in cellular mitochondria enables mammalian cells to live longer even when they’re infected with a substantial bacterial burden,” said Dario Zamboni, one of the corresponding authors of the article and a professor at the Ribeirão Preto Medical School (FMRP-USP).The study was carried out at the Center for Research on Inflammatory Diseases (CRID), one of FAPESP’s Research, Innovation and Dissemination Centers (RIDCs), in collaboration with Hayley Newton, a professor at Monash University in Australia. Funding was also provided by FAPESP via a project coordinated by Zamboni.“Essentially, we have uncovered a strategy used by C. burnetii to maintain cell health while replicating intensively. We found that its protein MceF redirects GPX4 to the mitochondria, where it acts as a potent antioxidant, detoxifying the infected cell and preventing cell components from aging, all the while allowing the bacterium to replicate,” explained Robson Kriiger Loterio, the lead author of the article, which was an outcome of his Ph.D. research.Cell biologistC. burnetii is responsible for causing a serious infection called Q fever, a zoonosis that is relatively common but diagnosed infrequently. According to the authors, agricultural outbreaks are “an increasingly significant economic and public health burden”.The bacterium induces atypical pneumonia in humans and coxiellosis in some animals, such as cattle, sheep, and goats. Zamboni explained that it is highly adapted to invade and control macrophages and monocytes – white blood cells that are part of the organism’s front-line immune defense – inhibiting the host’s responses to the infection.“The interest in studying this bacterium in depth lies precisely in its ability to subvert cell functions. Unlike other bacteria, which cause disease only when they multiply in large numbers, a single C. burnetii is enough to make a healthy person sick. Therefore, it efficiently modulates the cells it invades. We jokingly refer to it as a brilliant cell biologist due to its capability to modulate everything in host cells,” stated Zamboni.Additionally, C. burnetii replicates in cells for about a week, in contrast to Salmonella, which causes severe food poisoning and results in the death of host cells in less than 24 hours.“Studying C. burnetii provides insights into understanding cellular function. In this study, it helped us comprehend how to treat mitochondrial dysfunction and gain insights into programmed cell death in humans,” he added.To analyze the bacterium’s ability to subvert macrophages and directly impact mitochondria, the researchers conducted in vitro assays and experiments involving larvae of the Greater wax moth (Galleria mellonella). In the initial phase of the study, they explored over 80 novel proteins from C. burnettii with the potential to interact with host cells and alter their functionality. “We ended up focusing on MceF because it affects mitochondria directly, which play a crucial role in the process of cell death,” Zamboni concluded.The team plans to continue their research on two fronts, one focusing on gaining a deeper understanding of other proteins of interest, while the other involves biochemical studies to further comprehend how MceF influences GPX4.“What’s exciting about this research is that by examining a bacterium, we’re learning a lot about cell signaling, cell death, and innovative ways of reversing mitochondrial dysfunction. We don’t need to invent a new technique; the process already occurs during the bacterium’s interaction with host cells,” Zamboni concluded.Reference: “Coxiella co-opts the Glutathione Peroxidase 4 to protect the host cell from oxidative stress–induced cell death” by Robson K. Loterio, David R. Thomas, Warrison Andrade, Yi Wei Lee, Leonardo L. Santos, Danielle P. A. Mascarenhas, Thiago M. Steiner, Jéssica Chiaratto, Laura F. Fielden, Leticia Lopes, Lauren E. Bird, Gustavo H. Goldman, Diana Stojanovski, Nichollas E. Scott, Dario S. Zamboni and Hayley J. Newton, 28 August 2023, Proceedings of the National Academy of Sciences.
This discovery by researchers suggests potential new treatments for autoimmune diseases and cancer, as Coxiella burnetii, a Gram-negative intracellular bacterium, was found to produce a previously unidentified protein with antioxidant properties.Recently, scientists at the University of São Paulo, in collaboration with Australian colleagues, uncovered a unique bacterial protein with the ability to maintain human cell health even when the cells are heavily burdened by bacteria. This breakthrough has the potential to lead to the development of new treatments for various diseases related to mitochondrial dysfunction, including cancer and autoimmune disorders. Mitochondria, known as the cell’s powerhouses, are essential for producing the energy required for cellular biochemical reactions.The research article is published in the journal PNAS. The team scrutinized over 130 proteins released by Coxiella burnetii upon invading host cells and identified at least one protein capable of extending cell longevity by directly influencing mitochondria.Upon invading host cells, C. burnetii releases a previously unknown protein, named mitochondrial coxiella effector F (MceF) by the authors. MceF interacts with glutathione peroxidase 4 (GPX4), an antioxidant enzyme located in the mitochondria, to enhance mitochondrial function by promoting an antioxidizing effect, preventing cell damage and death that may occur when pathogens replicate inside mammalian cells.“C. burnetii employs various strategies to prevent the death of invaded cells and multiply inside them. One such strategy is the modulation of GPX4 by MceF, a mechanism that we discovered and reported in this article. The relocation of these proteins in cellular mitochondria enables mammalian cells to live longer even when they’re infected with a substantial bacterial burden,” said Dario Zamboni, one of the corresponding authors of the article and a professor at the Ribeirão Preto Medical School (FMRP-USP).The study was carried out at the Center for Research on Inflammatory Diseases (CRID), one of FAPESP’s Research, Innovation and Dissemination Centers (RIDCs), in collaboration with Hayley Newton, a professor at Monash University in Australia. Funding was also provided by FAPESP via a project coordinated by Zamboni.“Essentially, we have uncovered a strategy used by C. burnetii to maintain cell health while replicating intensively. We found that its protein MceF redirects GPX4 to the mitochondria, where it acts as a potent antioxidant, detoxifying the infected cell and preventing cell components from aging, all the while allowing the bacterium to replicate,” explained Robson Kriiger Loterio, the lead author of the article, which was an outcome of his Ph.D. research.Cell biologistC. burnetii is responsible for causing a serious infection called Q fever, a zoonosis that is relatively common but diagnosed infrequently. According to the authors, agricultural outbreaks are “an increasingly significant economic and public health burden”.The bacterium induces atypical pneumonia in humans and coxiellosis in some animals, such as cattle, sheep, and goats. Zamboni explained that it is highly adapted to invade and control macrophages and monocytes – white blood cells that are part of the organism’s front-line immune defense – inhibiting the host’s responses to the infection.“The interest in studying this bacterium in depth lies precisely in its ability to subvert cell functions. Unlike other bacteria, which cause disease only when they multiply in large numbers, a single C. burnetii is enough to make a healthy person sick. Therefore, it efficiently modulates the cells it invades. We jokingly refer to it as a brilliant cell biologist due to its capability to modulate everything in host cells,” stated Zamboni.Additionally, C. burnetii replicates in cells for about a week, in contrast to Salmonella, which causes severe food poisoning and results in the death of host cells in less than 24 hours.“Studying C. burnetii provides insights into understanding cellular function. In this study, it helped us comprehend how to treat mitochondrial dysfunction and gain insights into programmed cell death in humans,” he added.To analyze the bacterium’s ability to subvert macrophages and directly impact mitochondria, the researchers conducted in vitro assays and experiments involving larvae of the Greater wax moth (Galleria mellonella). In the initial phase of the study, they explored over 80 novel proteins from C. burnettii with the potential to interact with host cells and alter their functionality. “We ended up focusing on MceF because it affects mitochondria directly, which play a crucial role in the process of cell death,” Zamboni concluded.The team plans to continue their research on two fronts, one focusing on gaining a deeper understanding of other proteins of interest, while the other involves biochemical studies to further comprehend how MceF influences GPX4.“What’s exciting about this research is that by examining a bacterium, we’re learning a lot about cell signaling, cell death, and innovative ways of reversing mitochondrial dysfunction. We don’t need to invent a new technique; the process already occurs during the bacterium’s interaction with host cells,” Zamboni concluded.Reference: “Coxiella co-opts the Glutathione Peroxidase 4 to protect the host cell from oxidative stress–induced cell death” by Robson K. Loterio, David R. Thomas, Warrison Andrade, Yi Wei Lee, Leonardo L. Santos, Danielle P. A. Mascarenhas, Thiago M. Steiner, Jéssica Chiaratto, Laura F. Fielden, Leticia Lopes, Lauren E. Bird, Gustavo H. Goldman, Diana Stojanovski, Nichollas E. Scott, Dario S. Zamboni and Hayley J. Newton, 28 August 2023, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2308752120
Previously Unknown Protein Discovered to support Human Cell Health








/cdn.vox-cdn.com/uploads/chorus_asset/file/25806329/Screenshot_2024_12_26_at_5.43.25_PM.jpeg)



