Many organic molecules exist as “diastereomers” — molecules that experience the similar chemical construction however other spatial preparations in their atoms. In some circumstances, those slight structural variations may end up in important adjustments within the molecules’ purposes or chemical homes.As one instance, the most cancers drug doxorubicin could have heart-damaging unwanted side effects in a small proportion of sufferers. Alternatively, a diastereomer of the drug, referred to as epirubicin, which has a unmarried alcohol staff that issues in a special path, is far much less poisonous to coronary heart cells.“There are numerous examples like that during medicinal chemistry the place one thing that turns out small, corresponding to the placement of a unmarried atom in house, might in fact be actually profound,” says Alison Wendlandt, an affiliate professor of chemistry at MIT.Wendlandt’s lab is thinking about designing new equipment that may convert those molecules into other paperwork. Her staff could also be operating on identical equipment that may exchange a molecule into a special constitutional isomer — a molecule that has an atom or chemical staff positioned in a special spot, even if it has the similar chemical components as the unique.“You probably have a goal molecule and also you had to make it with out this kind of instrument, you would need to return to the start and make the entire molecule once more to get to the overall construction that you simply sought after,” Wendlandt says.Those equipment too can lend themselves to making solely new molecules that may well be tricky and even unimaginable to construct the use of conventional chemical synthesis tactics.“We’re thinking about a large suite of selective transformations, the purpose being to make the most important have an effect on on how it’s possible you’ll envision creating a molecule,” she says. “If you’ll be able to open up get admission to to the interconversion of molecular constructions, you’ll then suppose totally otherwise about how you may make a molecule.”From math to chemistryAs the daughter of 2 geologists, Wendlandt discovered herself immersed in science from a tender age. Either one of her folks labored on the Colorado Faculty of Mines, and circle of relatives holidays continuously concerned journeys to attention-grabbing geological formations.In highschool, she discovered math extra interesting than chemistry, and he or she headed to the College of Chicago with plans to primary in arithmetic. Alternatively, she quickly had 2d ideas, after encountering summary math.“I used to be just right at calculus and the type of math you wish to have for engineering, but if I were given to school and I encountered topology and N-dimensional geometry, I noticed I don’t in fact have the talents for summary math. At that time I turned into a little bit bit extra open-minded about what I sought after to check,” she says.Although she didn’t suppose she favored chemistry, an natural chemistry route in her sophomore 12 months modified her thoughts.“I beloved the problem-solving side of it. I’ve an overly, very dangerous reminiscence, and I couldn’t memorize my means in the course of the elegance, so I needed to simply be told it, and that used to be simply so a laugh,” she says.As a chemistry primary, she started operating in a lab thinking about “general synthesis,” a analysis space that comes to growing methods to synthesize a fancy molecule, continuously a herbal compound, from scratch.Even though she beloved natural chemistry, a lab twist of fate — an explosion that injured a pupil in her lab and resulted in brief listening to loss for Wendlandt — made her hesitant to pursue it additional. When she implemented to graduate faculties, she determined to enter a special department of chemistry — chemical biology. She studied at Yale College for a few years, however she discovered that she didn’t revel in that form of chemistry and left after receiving a grasp’s stage.She labored in a lab on the College of Kentucky for a couple of years, then implemented to graduate college once more, this time on the College of Wisconsin. There, she labored in an natural chemistry lab, finding out oxidation reactions which may be used to generate prescribed drugs or different helpful compounds from petrochemicals.After completing her PhD in 2015, Wendlandt went to Harvard College for a postdoc, operating with chemistry professor Eric Jacobsen. There, she turned into interested by selective chemical reactions that generate a specific isomer, and started finding out catalysts that might carry out glycosylation — the addition of sugar molecules to different molecules — at explicit websites.Modifying moleculesSince becoming a member of the MIT school in 2018, Wendlandt has labored on growing catalysts that may convert a molecule into its reflect symbol or an isomer of the unique.In 2022, she and her scholars evolved a device referred to as a stereo-editor, which will adjust the association of chemical teams round a central atom referred to as a stereocenter. This editor is composed of 2 catalysts that paintings in combination to first upload sufficient power to take away an atom from a stereocenter, then exchange it with an atom that has the other orientation. That power enter comes from a photocatalyst, which converts captured gentle into power.“You probably have a molecule with an present stereocenter, and you wish to have the opposite enantiomer, generally you would need to get started over and make the opposite enantiomer. However this new means tries to interconvert them immediately, so it offers you a mind-set about molecules as dynamic,” Wendlandt says. “You must generate any form of 3-dimensional construction of that molecule, after which in an impartial step later, it is advisable to totally reorganize the 3-d construction.”She has additionally evolved equipment that may convert commonplace sugars corresponding to glucose into different isomers, together with allose and different sugars which can be tricky to isolate from herbal assets, and equipment that may create new isomers of steroids and alcohols. She is now operating on techniques to transform 6-membered carbon rings to seven or 8-membered rings, and so as to add, subtract, or exchange one of the vital chemical teams connected to the rings.“I’m interested by growing normal equipment that can let us interconvert static constructions. So, that can be taking a undeniable practical staff and transferring it to any other a part of the molecule solely, or taking massive rings and making them small rings,” she says. “As a substitute of pondering of molecules that we compile as static, we’re fascinated by them now as probably dynamic constructions, which might exchange how we take into accounts making natural molecules.”This manner additionally opens up the potential of growing emblem new molecules that haven’t been noticed prior to, Wendlandt says. This might be helpful, as an example, to create drug molecules that engage with a goal enzyme in simply the fitting means.“There’s an enormous quantity of chemical house that’s nonetheless unknown, atypical chemical house that simply has no longer been made. That’s partly as a result of perhaps nobody has been interested by it, or as it’s simply too exhausting to make that exact factor,” she says. “These kind of equipment provide you with get admission to to isomers which can be perhaps no longer simply made.”





:max_bytes(150000):strip_icc()/StephanieBrownnewheadshot-e5ca9ba2a404491384e9300a7871f190.jpg)








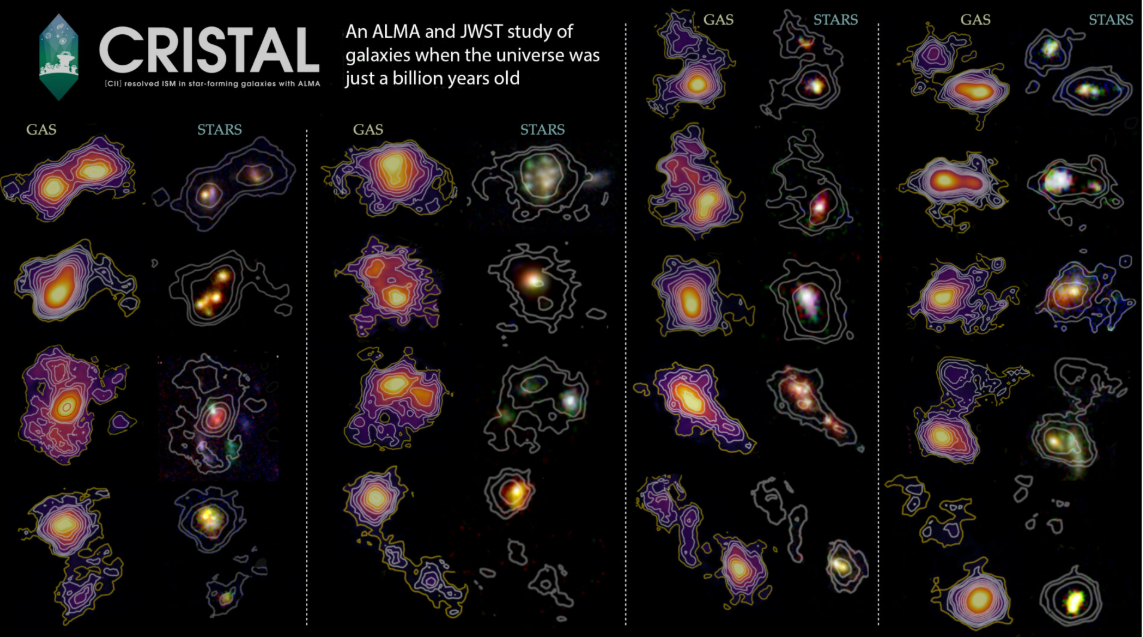
 The COSMOS-Internet venture has created probably the most
The COSMOS-Internet venture has created probably the most