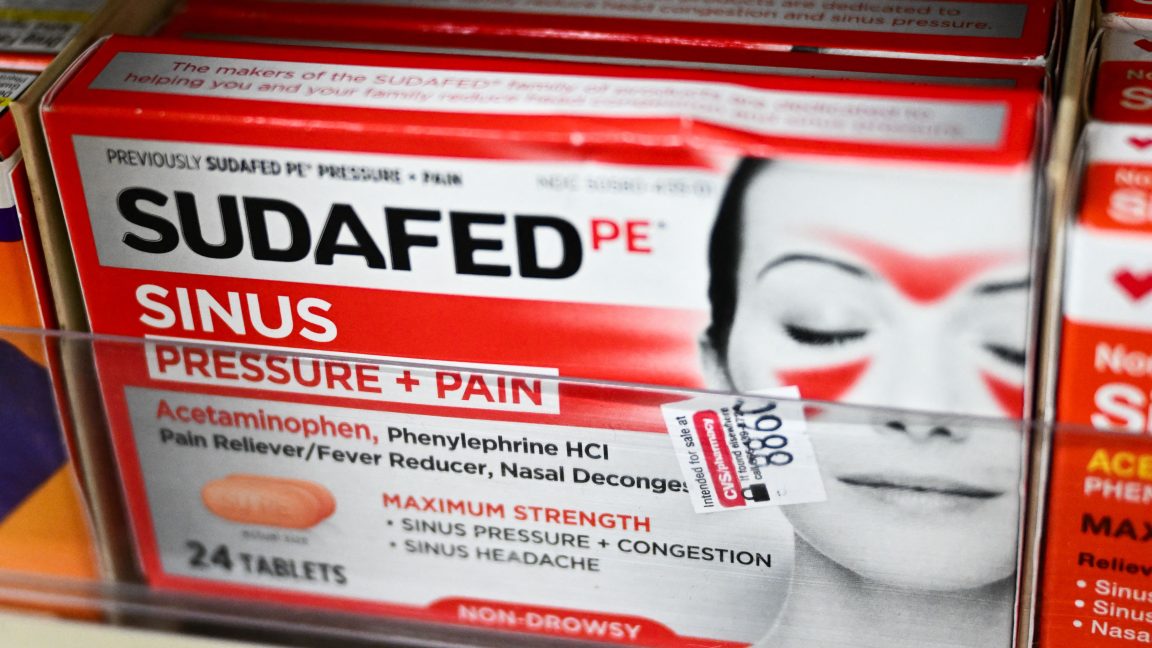
In a long-sought transfer, the Meals and Drug Management on Thursday officially started the method of forsaking oral doses of a commonplace over the counter decongestant, which the company concluded ultimate 12 months isn’t efficient at relieving stuffy noses.
In particular, the FDA issued a proposed order to take away oral phenylephrine from the checklist of substances that drugmakers can come with in over the counter merchandise—often referred to as the OTC monograph. As soon as got rid of, drug makers will not be capable to come with phenylephrine in merchandise for the transient aid nasal congestion.
“It’s the FDA’s position to make certain that medication are secure and efficient,” Patrizia Cavazzoni, director of the FDA’s Heart for Drug Analysis and Analysis, mentioned in a observation. “In accordance with our assessment of to be had information and in keeping with the recommendation of the advisory committee, we’re taking this subsequent step within the procedure to suggest taking away oral phenylephrine as a result of it’s not efficient as a nasal decongestant.”
For now, the order is only a proposal. The FDA will open up a public remark duration, and if no feedback can sway the FDA’s earlier conclusion that the drug is pointless, the company will make the order ultimate. Drugmakers gets a grace duration to reformulate their merchandise.
Reviewed evaluations
The slow-moving abandonment of phenylephrine is years within the making. The decongestant used to be in the beginning authorized by means of the FDA again in 1976, however it got here to prominence after 2006. That used to be the 12 months when the “Struggle Methamphetamine Epidemic Act of 2005” got here into impact, and pseudoephedrine—the principle element of Sudafed—moved in the back of the drugstore counter to stay it from getting used to make methamphetamine. With pseudoephedrine out of simple succeed in at drugstores, phenylephrine was the main over the counter decongestant. And researchers had questions.
In 2007, an FDA panel reevaluated the drug, which allegedly works by means of shrinking blood vessels within the nasal passage, opening up the airway. Whilst the panel upheld the drug’s approval, it concluded that extra research had been wanted for a complete evaluate. After that, 3 huge, moderately designed research had been performed—two by means of Merck for the remedy of seasonal allergic reactions and one by means of Johnson & Johnson for the remedy of the average chilly. All 3 discovered no important distinction between phenylephrine and a placebo.