The Gentleman Report
—
Beginning January 1, a drug that hundreds of sufferers rely on to lend a hand them breathe will disappear from pharmacy cabinets, and docs are involved sufferers can have delays switching to choices and getting them lined through insurance coverage.
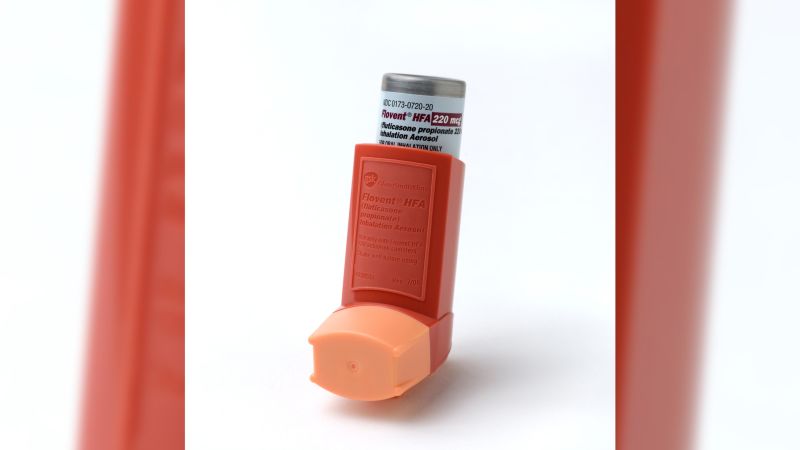
Producer GSK has mentioned it’s discontinuing the branded bronchial asthma inhaler Flovent, and as an alternative is making an “approved generic” model, which is similar however with out the similar branding.
Physicians who deal with sufferers with bronchial asthma say the approved generic will paintings simply in addition to the branded drug, however it doesn’t seem to be lined as broadly through insurers. That can imply sufferers must download new prescriptions and kind out protection hurdles on the top of respiration virus season.
“This drugs has been probably the most frequently used inhaled drugs for the previous 25 or 30 years,” mentioned Dr. Robyn Cohen, a pediatric pulmonologist at Boston Clinical Middle. “It’s the person who, overwhelmingly, pediatricians succeed in for after they come to a decision that their affected person wishes a day-to-day preventive drugs. … The truth that it’s being discontinued goes to be an enormous surprise to the gadget for sufferers, for households and for docs.”
Medical doctors are urging sufferers to do so now to make sure they’ve were given their drugs heading into the brand new yr and advocacy teams had been looking to get the phrase out.
However the tale of why Flovent is disappearing, and the loss of insurance plans for its ostensibly equivalent alternative, touches on one of the most most complicated sides of American well being care and drug pricing.
A spokeswoman for GSK mentioned the corporate is making the alternate “as a part of our dedication to be bold for sufferers.”
She famous the corporate offered the approved generics of Flovent HFA, an inhalation aerosol, and Flovent Diskus, an inhalation powder, in Would possibly 2022 and October 2023, and that, due to this fact, it might discontinue production the branded variations in america on January 1, 2024.
The approved generics, she mentioned, “will supply sufferers in the United States with doubtlessly lower price choices of those medically essential merchandise.”
Mavens who practice the trade each on Wall Side road and in academia, regardless that, indicate GSK is making the transfer at exactly the time a metamorphosis in Medicaid rebates may just purpose the corporate to need to pay huge consequences on account of value will increase on Flovent over plenty of years.
The criminal alternate entering impact at the first of the yr gets rid of a cap on Medicaid rebates that businesses are required to pay in the event that they carry the cost of medications greater than inflation.
“Flovent Diskus has been available on the market since 2000 and Flovent HFA since 2004, and GSK has hiked the cost on each merchandise a large number of occasions since their release,” Dr. William Feldman, an affiliate doctor within the Department of Pulmonary and Essential Care Drugs at Brigham and Girls’s Health facility who research bronchial asthma medication, advised The Gentleman Report. “Those are exactly this type of medication that can be suffering from the brand new coverage getting rid of the Medicaid rebate cap.”
Till now, the rebates have been capped on the overall value of a drug, so producers would by no means pay greater than a drug prices again to Medicaid.
However beneath a provision within the 2021 American Rescue Plan Act, that prohibit was once got rid of, and beginning January 1, 2024, medication which have been matter to huge value will increase through the years may just finally end up incurring rebates to Medicaid which might be more than their value — which means pharmaceutical corporations would promote the ones medication to Medicaid at a loss.
“Clearly pharma doesn’t wish to be promoting at a loss on anything else in its portfolio,” mentioned Andrew Baum, an analyst who covers the inventory of GSK and different pharmaceutical corporations for the monetary company Citi. “So it seeks to evade affect through, one: discontinuation; two: approved generic.”
A certified generic, Baum advised The Gentleman Report, is seen as a separate product, “however nonetheless allows pharma to gather one of the most economics.”
Or, put in a different way, it’s the similar product with out the branding and in addition with out the historical past of value will increase that would depart the drugs at risk of such huge rebates to Medicaid.
Consistent with information from GoodRx, the cost of branded Flovent has long past up about 47% since 2014.
Different drugmakers have made adjustments forward of the January 1 rebate cap elimination as smartly; makers of insulin this yr introduced main value cuts — of 70% or extra — on their merchandise, a transfer analysts estimate will save them masses of thousands and thousands of greenbacks a yr.
The approved generic technique GSK is using “is some way, widely talking, to maximise the profitability of the product in query,” mentioned David Amsellem, a monetary analyst who covers the trade at funding company Piper Sandler.
He famous there are recently no different generic variations of Flovent licensed through the FDA.
GSK did value the approved generic less than branded Flovent; one bundle of Flovent HFA within the 110 microgram dose, for instance, prices $273.83, about 50% greater than the $177.99 wholesale acquisition price of its approved generic counterpart, consistent with costs the corporate shared with The Gentleman Report. The wholesale acquisition price is the cost prior to insurance coverage and rebates.
However CVS Caremark, a significant pharmacy receive advantages supervisor that determines which medications are lined through insurance coverage for its contributors, is giving preferential placement to some other branded inhaler, Pulmicort, on its formulary, as an alternative of the approved generic variations of Flovent.
“On this case, the approved generics have been dearer than the emblem title medicines,” a CVS spokesman advised The Gentleman Report. He famous that’s in keeping with internet costs, quite than the wholesale acquisition price, which means Pulmicort might be more economical on account of rebates its producer, AstraZeneca, can pay to acquire higher insurance plans.
The truth that insurance coverage aren’t widely overlaying the approved generic of Flovent, mentioned BMC’s Cohen, “signifies that sufferers are going to wish to get a brand spanking new prescription for an absolutely other drugs in the midst of the worst imaginable time of yr, which is the wintry weather respiration virus season.”
For sufferers with continual bronchial asthma, Cohen mentioned, Flovent has been probably the most frequently used day-to-day preventive anti inflammatory drugs for many years. It shrinks swelling within the airlines and decreases the frame’s exaggerated reaction to triggers that make it onerous to respire.
Throughout chilly and flu season, she mentioned, it turns into much more an important to have that day-to-day drugs.
“Flu, Covid, RSV — these kinds of circulating viruses which might be going round at the moment — are some of the largest, if no longer the largest, triggers for bronchial asthma assaults in youngsters,” Cohen mentioned. “That is what results in youngsters being within the emergency room.”
Cohen mentioned she’s involved that sufferers, in addition to physicians and pharmacists, don’t know this variation with Flovent is coming, they usually wish to act now to figure out choices and resolve insurance plans.
Get The Gentleman Report Well being’s weekly e-newsletter
For some teams, the choices are extra restricted. For sufferers with a extra uncommon inflammatory situation, referred to as eosinophilic esophagitis, Flovent HFA is among the maximum frequently prescribed topical steroids, and different medications don’t have as a lot information supporting their use within the situation, mentioned Dr. Erin Syverson, an attending doctor within the Department of Gastroenterology, Hepatology and Vitamin at Boston Youngsters’s Health facility.
As EoE impacts the esophagus, sufferers swallow the drugs as an alternative of respiring it in, and it could possibly tame the irritation that may purpose ache with swallowing or meals getting caught, requiring procedures to take away it. In youngsters, Syverson mentioned, EoE can result in recurrent vomiting, heartburn, abdominal aches, and bother making development beginning cast meals, and Flovent can lend a hand stay the situation beneath keep watch over.
“With the discontinuation arising, I concern it’s going to simply be another hurdle for this affected person inhabitants that already has very restricted medicines to be had to them,” Syverson advised The Gentleman Report. “I don’t know what January goes to be like, however I’m apprehensive.”
The Gentleman Report’s Tami Luhby contributed to this document.