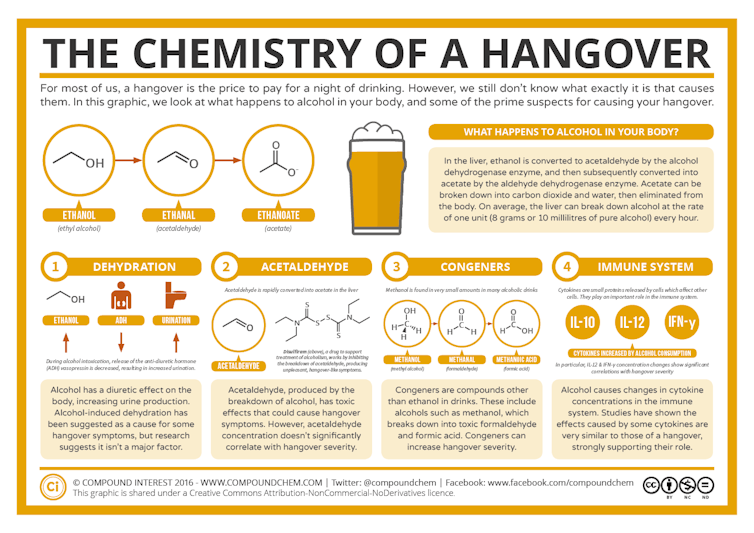
This text has been reviewed in line with Science X’s editorial procedure
and insurance policies.
Editors have highlighted the next attributes whilst making sure the content material’s credibility:
fact-checked
peer-reviewed newsletter
relied on supply
proofread
Adequate!
Credit score: Pixabay/CC0 Public Area
× shut
Credit score: Pixabay/CC0 Public Area
The chemical trade has lengthy been shadowed via unwelcome photographs of billowing smokestacks and pipes discharging poisonous effluent. Trendy production practices have achieved a lot to mitigate the trade’s environmental affect, however there stays room for growth.
Making chemistry extra environmentally pleasant is a keenness and main analysis focal point for Caltech’s Karthish Manthiram, professor of chemical engineering and chemistry, and a William H. Harm Student.
In a paper showing within the magazine Science, Manthiram’s lab describes the advance of a catalyst for generating a broadly used chemical feedstock with out the poisonous and perilous chemical compounds generally required for its manufacturing.
That chemical feedstock, propylene oxide, is an natural compound utilized in quite a lot of programs, together with production foams, plastics, and antifreeze, in addition to for disinfection and sterilization. Historically, propylene oxide is produced via reacting propylene with both hypochlorous acid or hydrogen peroxide. Each and every has its personal downside.
“With hypochlorous acid, you find yourself with a chloride aspect product that you just discharge out into the surroundings. Because of this, there are fewer and less allows being granted to permit for crops that use the hypochlorous acid procedure,” Manthiram says. “That has pressured folks to shift towards peroxide-based processes, however you may have this massive protection problem. Any time you may have hydrogen peroxide in touch with natural compounds, there’s a looming danger of explosions.”
The crowd’s objective was once to expand a protected manner for propylene epoxide manufacturing that didn’t produce an environmental discharge or have a big carbon footprint. Manthiram says the workforce started via on the lookout for a catalyst able to generating propylene epoxide the use of the oxygen atom present in a water molecule. The one aspect product could be hydrogen gasoline, which can be utilized as a gasoline or in production different chemical compounds.
“The entire premise was once that water is protected,” he says. “It does not provide an intrinsic protection danger, and there is not any environmentally damaging aspect product from the method. As a substitute, you are making hydrogen, which is one thing that we want to be making extra of one day. That is the place we began.”
The crowd narrowed in on two catalysts: platinum oxide and palladium oxide. Each carried out the response the workforce sought after, however now not sufficiently neatly to be helpful. Platinum oxide produced propylene epoxide at prime charges, however messily, growing many undesirable aspect merchandise. Against this, palladium oxide produced propylene epoxide with fewer aspect merchandise, nevertheless it did so quite slowly.
Manthiram says the answer was once to mix the 2 catalysts.
“Striking the 2 in combination if truth be told ended up fixing the issue,” says Minju Chung, lead writer and previous postdoctoral student on the Georgia Institute of Generation, now with MIT. “Then we spent a large number of time figuring out why that combination works higher. It is not an easy clarification.”
The use of X-ray absorption spectroscopy (a method that may divulge the atomic and digital construction of fabrics via bombarding them with X-rays), the researchers made up our minds that during a mix of platinum oxide and palladium oxide, the platinum exists in a state that makes it a extra environment friendly catalyst.
“It seems that some of the dramatic results of going from platinum oxide to palladium–platinum oxide is that you’ll stabilize the platinum in the next oxidation state,” Manthiram says. “When in the next oxidation state, the oxygen hooked up to the platinum is extra disadvantaged of electrons, making it extra reactive with the electron-rich propylene. We see thru a complete sequence of experiments that stabilizing platinum in the next oxidation state results in considerably stepped forward charges and efficiencies of propylene epoxidation.”
The use of the brand new catalyst, the speed of propylene oxide manufacturing is 10 occasions upper than what had in the past been accomplished, and the potency is higher via 13 %, Manthiram says.
Manthiram says long term analysis will focal point on checking out the catalyst to peer how it may be taken from a laboratory setup into business settings. That may require analyses that read about how lengthy the catalyst lasts earlier than it degrades and the way neatly it plays at greater scales, in addition to the advance of a procedure for putting off the propylene epoxide from the gadget as it’s produced.
“It is time to graduate this subject matter from this basic science context,” he says. “That is going to be actually illuminating to us for us as a result of it is going to display us what are the following issues that we will have to paintings on.”
The paper describing the paintings, “Direct propylene epoxidation by way of water activation over Pd-Pt electrocatalysts,” seems within the January 4 factor of Science.
Additional info:
Minju Chung et al, Direct propylene epoxidation by way of water activation over Pd-Pt electrocatalysts, Science (2024). DOI: 10.1126/science.adh4355
Magazine data:
Science