HYDERABAD: Bharat Biotech, primarily based in Hyderabad, has began scientific trials of MTBVAC, the arena’s first Mycobacterium tuberculosis vaccine derived from a human supply, in India.
The scientific trial will evaluation the vaccine’s protection, immunogenicity and efficacy. It’s being evolved via Spanish biopharma participant Biofabri in collaboration with Bharat Biotech.
Assets mentioned Bharat Biotech can have unique world production rights for the reside attenuated vaccine this is being evolved for newborns, kids and adults.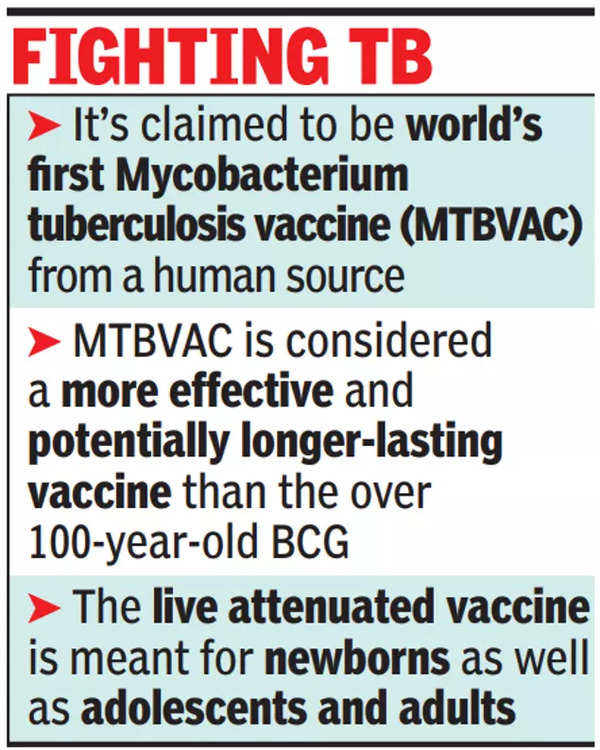
MTBVAC is being evolved as a more practical and doubtlessly longer-lasting vaccine than BCG vaccine, this is over 100 years outdated. It’s going to be for newborns, in addition to for the prevention of TB in adults and kids for whom there’s these days no efficient vaccine, assets mentioned.
The prevailing BCG vaccine is an attenuated variant of the bovine TB pathogen and has restricted impact on pulmonary TB, which is liable for the illness transmission.
Learning the protection, immunogenicity and efficacy of MTBVAC in probably the most populated nation on the earth and one with the easiest selection of TB circumstances is essential to proceed the development of the vaccine, which has gone through over 3 many years of study.
Biofabri CEO Esteban Rodriguez referred to as it a “large step to check in adults and kids within the nation the place 28% of the arena’s TB circumstances gather”.
Terming the scientific trials in India as a large step within the function to broaden TB vaccines to stop the life-threatening illness in adults and kids, Bharat Biotech govt chairman Dr Krishna Ella mentioned, “Our quest for a more practical vaccine in opposition to TB gained a large spice up lately, with scientific trials in India.”
MTBVAC is these days the one TB vaccine present process scientific trials according to a genetically changed type of the pathogen, Mycobacterium tuberculosis, that, not like BCG, accommodates all of the antigens found in lines that infect people.
The vaccine used to be evolved in Spain’s College of Zaragoza’s laboratory in collaboration with Dr Brigitte Gicquel of the Pasteur Institute in Paris. Biofabri is Zaragoza College’s commercial spouse.
The vaccine has not too long ago finished a Section-2 dose discovering trial. A double-blind, managed Section-3 scientific trial in newborns used to be began in 2023 in South Africa, Madagascar and Senegal to check it with the one TB vaccine in use, the BCG vaccine.
Bharat Biotech starts scientific trials of more practical TB vaccine in India | India Information – Occasions of India