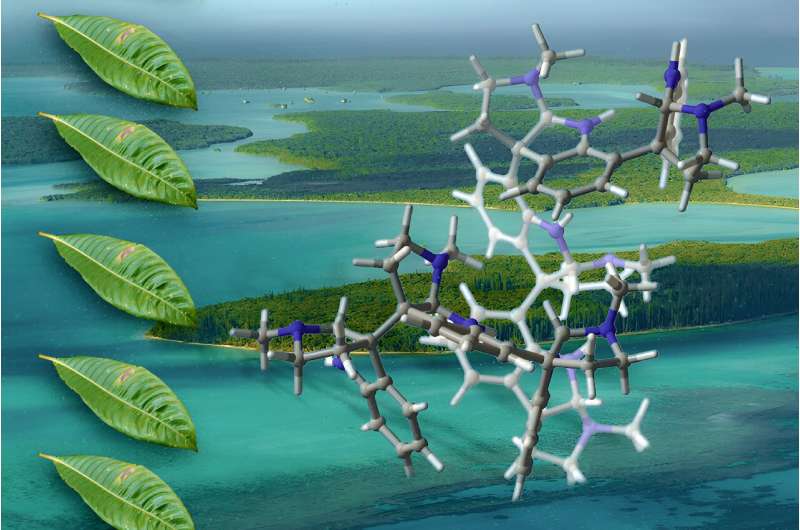
The oligocyclotryptamines had been in the beginning remoted from Psychotria leaves in New Caledonia. Credit score: Jose-Luis Olivares, MIT; molecule supplied through Scott et al.
MIT chemists have advanced a brand new method to synthesize advanced molecules that had been in the beginning remoted from crops and may hang attainable as antibiotics, analgesics, or most cancers medicine.
Those compounds, referred to as oligocyclotryptamines, encompass a couple of tricyclic substructures known as cyclotryptamine, fused in combination through carbon–carbon bonds. Handiest small amounts of those compounds are naturally to be had, and synthesizing them within the lab has confirmed tricky. The MIT staff got here up with some way so as to add tryptamine-derived parts to a molecule separately, in some way that permits the researchers to exactly compile the rings and keep watch over the 3-D orientation of each and every part in addition to the overall product.
“For lots of of those compounds, there hasn’t been sufficient subject matter to do an intensive assessment in their attainable. I am hopeful that gaining access to those compounds in a competent method will permit us to do additional research,” says Mohammad Movassaghi, an MIT professor of chemistry and the senior writer of the brand new find out about.
Along with permitting scientists to synthesize oligocyclotryptamines present in crops, this manner may be used to generate new variants that can have even higher medicinal houses, or molecular probes that may lend a hand to show their mechanism of motion.
Tony Scott, Ph.D. is the lead writer of the paper, which seems within the Magazine of the American Chemical Society.
Fusing rings
Oligocyclotryptamines belong to a category of molecules known as alkaloids—nitrogen-containing natural compounds produced basically through crops. No less than 8 other oligocyclotryptamines were remoted from a genus of flowering crops referred to as Psychotria, maximum of that are present in tropical forests.
For the reason that Nineteen Fifties, scientists have studied the construction and synthesis of dimeric cyclotryptamines, that have two cyclotryptamine subunits. During the last two decades, vital growth has been made characterizing and synthesizing dimers and different smaller family members. On the other hand, no person has been ready to synthesize the most important oligocyclotryptamines, that have six or seven rings fused in combination.
Some of the hurdles in synthesizing those molecules is a step that calls for formation of a bond between a carbon atom of 1 tryptamine-derived subunit to a carbon atom of the following subunit. The oligocyclotryptamines have two varieties of those linkages, each containing a minimum of one carbon atom that has bonds with 4 different carbons. That additional bulk makes the ones carbon atoms much less available to go through reactions, and controlling the stereochemistry—the orientation of the atoms across the carbon—at a lot of these junctures poses a vital problem.
For a few years, Movassaghi’s lab has been creating tactics to shape carbon-carbon bonds between carbon atoms which are already crowded with different atoms. In 2011, they devised one way that comes to reworking the 2 carbon atoms into carbon radicals (carbon atoms with one unpaired electron) and directing their union. To create those radicals, and information the paired union to be totally selective, the researchers first connect each and every of the centered carbon atoms to a nitrogen atom; those two nitrogen atoms bind to one another.
When the researchers shine positive wavelengths of sunshine at the substrate containing the 2 fragments connected by means of the 2 nitrogen atoms, it reasons the 2 atoms of nitrogen to break free as nitrogen fuel, leaving in the back of two very reactive carbon radicals in shut proximity that sign up for in combination virtually instantly. This sort of bond formation has additionally allowed the researchers to keep watch over the molecules’ stereochemistry.
Movassaghi demonstrated this manner, which he calls diazene-directed meeting, through synthesizing different varieties of alkaloids, together with the communesins. Those compounds are present in fungi and consist of 2 ring-containing molecules, or monomers, joined in combination. Later, Movassaghi started the use of this solution to fuse greater numbers of monomers, and he and Scott in the end became their consideration to the most important oligocyclotryptamine alkaloids.
The synthesis that they advanced starts with one molecule of cyclotryptamine spinoff, to which further cyclotryptamine fragments with right kind relative stereochemistry and place selectivity are added, separately. Each and every of those additions is made imaginable through the diazene-directed procedure that Movassaghi’s lab prior to now advanced.
“The explanation why we are fascinated with that is that this unmarried resolution allowed us to head after a couple of objectives,” Movassaghi says. “That very same path supplies us a way to a couple of participants of the herbal product circle of relatives as a result of through extending the iteration yet one more cycle, your resolution is now carried out to a brand new herbal product.”
‘A excursion de power’
The use of this manner, the researchers had been ready to create molecules with six or seven cyclotryptamine rings, which hasn’t ever been finished earlier than.
“Researchers international were looking for a method to make those molecules, and Movassaghi and Scott are the primary to drag it off,” says Seth Herzon, a professor of chemistry at Yale College, who was once now not concerned within the analysis. Herzon described the paintings as “a excursion de power in natural synthesis.”
Now that the researchers have synthesized those naturally happening oligocyclotryptamines, they will have to be capable of generate sufficient of the compounds that their attainable healing job may also be extra completely investigated.
They will have to additionally be capable of create novel compounds through switching in moderately other cyclotryptamine subunits, Movassaghi says.
“We can proceed to make use of this very actual method of including those cyclotryptamine gadgets to collect them in combination into advanced programs that experience now not been addressed but, together with derivatives that might doubtlessly have progressed houses,” he says.
Additional information:
Tony Z. Scott et al, Unified, Biosynthesis-Impressed, Utterly Stereocontrolled Overall Synthesis of All Best-Order [n + 1] Oligocyclotryptamine Alkaloids, Magazine of the American Chemical Society (2024). DOI: 10.1021/jacs.4c07705
Supplied through
Massachusetts Institute of Era
This tale is republished courtesy of MIT Information (internet.mit.edu/newsoffice/), a well-liked web site that covers information about MIT analysis, innovation and educating.
Quotation:
Chemists synthesize plant-derived molecules that hang attainable as prescribed drugs (2024, August 12)
retrieved 13 August 2024
from
This file is matter to copyright. Except any honest dealing for the aim of personal find out about or analysis, no
phase is also reproduced with out the written permission. The content material is supplied for info functions simplest.