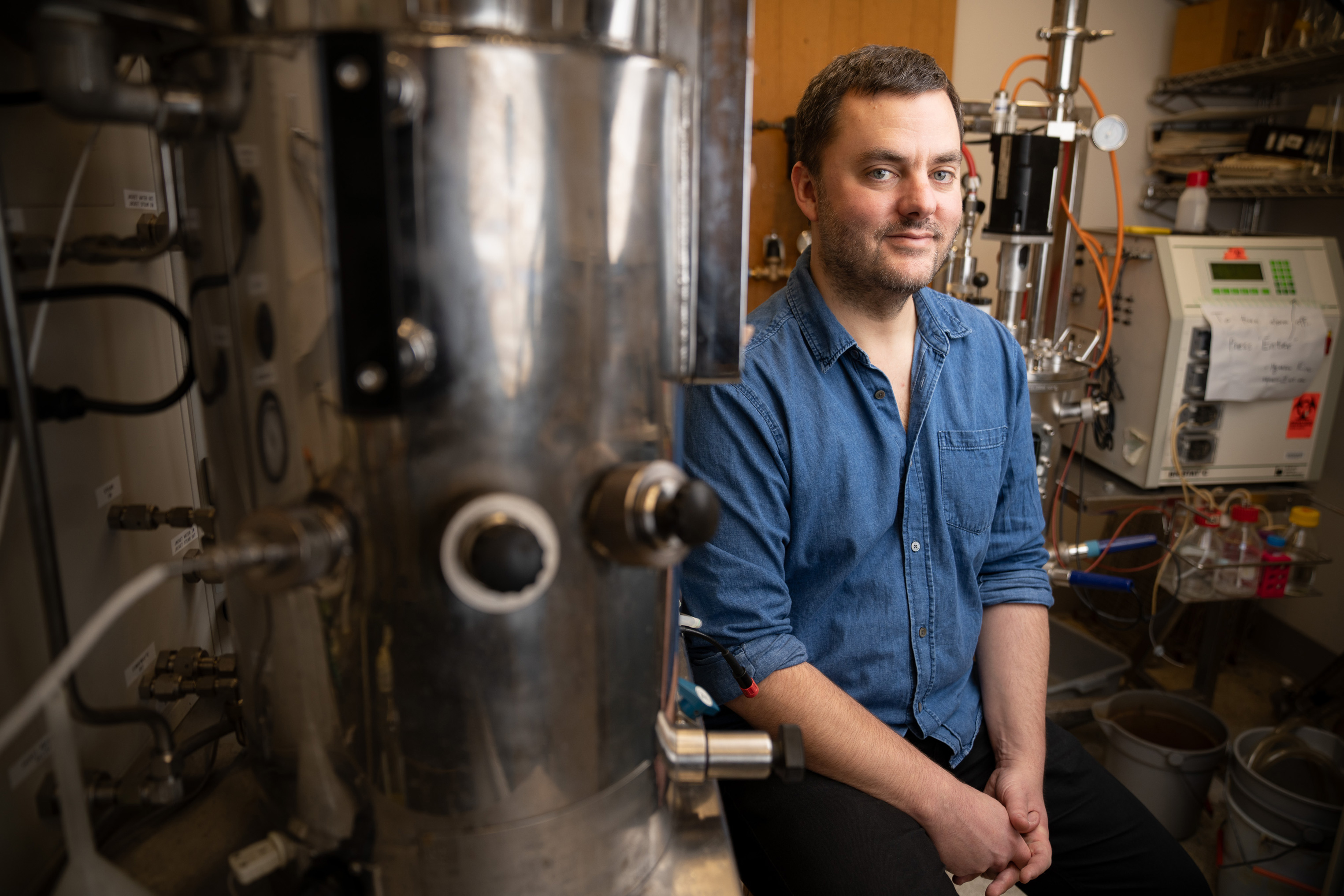
To assist to find answers to the planet’s local weather disaster, MIT Affiliate Professor Daniel Suess is having a look to Earth’s historic previous.Early within the evolution of existence, cells received the power to accomplish reactions reminiscent of shifting electrons from one atom to every other. Those reactions, which assist cells to construct carbon-containing or nitrogen-containing compounds, depend on specialised enzymes with clusters of metallic atoms.By way of finding out extra about how the ones enzymes paintings, Suess hopes to ultimately devise new techniques to accomplish elementary chemical reactions that would assist seize carbon from the ambience or permit the improvement of different fuels.“We need to to find a way of rewiring society in order that we aren’t simply depending on huge reserves of decreased carbon, fossil fuels, and burning them the usage of oxygen,” he says. “What we’re doing is we’re having a look backward, as much as 1000000000 years prior to oxygen and photosynthesis got here alongside, to peer if we will establish the chemical rules that underlie processes that aren’t reliant on burning carbon.”His paintings may just additionally make clear different essential mobile reactions such because the conversion of nitrogen fuel to ammonia, which may be the important thing step within the manufacturing of artificial fertilizer.Exploring chemistrySuess, who grew up in Spokane, Washington, was occupied with math at a tender age, however ended up majoring in chemistry and English at Williams Faculty, which he selected in response to its interesting collection of classes.“I used to be occupied with colleges that had been extra targeted at the liberal arts fashion, Williams being a kind of. And I simply idea they’d the correct mix of actually fascinating classes and freedom to take categories that you simply sought after,” he says. “I went in no longer anticipating to primary in chemistry, however then I actually loved my chemistry categories and chemistry academics.”In his categories, he explored all facets of chemistry and located all of them interesting.“I appreciated natural chemistry, as a result of there’s an emphasis on making issues. And I appreciated bodily chemistry as a result of there was once an try to have no less than a semiquantitative manner of figuring out the sector. Bodily chemistry describes one of the most maximum essential trends in science within the twentieth century, together with quantum mechanics and its utility to atoms and molecules,” he says.After school, Suess got here to MIT for graduate faculty and started operating with chemistry professor Jonas Peters, who had just lately arrived from Caltech. A few years later, Peters ended up shifting again to Caltech, and Suess adopted, proceeding his PhD thesis analysis on new techniques to synthesize inorganic molecules.His mission interested in molecules that include a metallic reminiscent of iron or cobalt sure to a nonmetallic crew referred to as a ligand. Inside those molecules, the metallic atom usually pulls in electrons from the ligand. On the other hand, the molecules Suess labored on had been designed in order that the metallic would surrender its personal electrons to the ligand. Such molecules can be utilized to hurry up tough reactions that require breaking very sturdy bonds, just like the nitrogen-nitrogen triple bond in N2.Throughout a postdoc on the College of California at Davis, Suess switched gears and started operating on biomolecules — in particular, metalloproteins. Those are protein enzymes that experience metals tucked into their lively websites, the place they assist to catalyze reactions.Suess studied how cells synthesize the metal-containing lively websites in those proteins, that specialize in an enzyme known as iron-iron hydrogenase. This enzyme, discovered basically in anaerobic micro organism, together with some that are living within the human digestive tract, catalyzes reactions involving the switch of protons and electrons. In particular, it will probably mix two protons and two electrons to make H2, or can carry out the opposite response, breaking H2 into protons and electrons.“That enzyme is actually essential as a result of a large number of mobile metabolic processes both generate extra electrons or require extra electrons. Should you generate extra electrons, they have got to head someplace, and one resolution is to place them on protons to make H2,” Suess says.World scale reactionsSince becoming a member of the MIT school in 2017, Suess has persisted his investigations of metalloproteins and the reactions that they catalyze.“We’re occupied with global-scale chemical reactions, that means they’re happening at the microscopic scale however taking place on an enormous scale,” he says. “They have an effect on the planet and feature decided what the molecular composition of the biosphere is and what it’s going to be.”Photosynthesis, which emerged round 2.4 billion years in the past, has had the largest have an effect on at the setting, filling it with oxygen, however Suess specializes in reactions that cells started the usage of even previous, when the ambience lacked oxygen and mobile metabolism may just no longer be pushed by way of breathing.Many of those historic reactions, which might be nonetheless utilized by cells nowadays, contain a category of metalloproteins known as iron-sulfur proteins. Those enzymes, which might be present in all kingdoms of existence, are all for catalyzing lots of the maximum tough reactions that happen in cells, reminiscent of forming carbon radicals and changing nitrogen to ammonia.To check the metalloenzymes that catalyze those reactions, Suess’s lab takes two other approaches. In a single, they invent artificial variations of the proteins that can comprise fewer metallic atoms, which permits for higher regulate over the composition and form of the protein, making them more straightforward to review.In every other way, they use the herbal model of the protein however exchange one of the crucial metallic atoms with an isotope that makes it more straightforward to make use of spectroscopic tactics to research the protein’s construction.“That permits us to review each the bonding within the resting state of an enzyme, in addition to the bonding and constructions of response intermediates that you’ll handiest signify spectroscopically,” Suess says.Working out how enzymes carry out those reactions may just assist researchers to find new techniques to take away carbon dioxide from the ambience by way of combining it with different molecules to create better compounds. Discovering different ways to transform nitrogen fuel to ammonia may just even have a large have an effect on on greenhouse fuel emissions, because the Haber Bosch procedure now used to synthesize fertilizer produces calls for massive quantities of power.“Our number one focal point is on figuring out the flora and fauna, however I believe that as we’re having a look at other ways to cord organic catalysts to do environment friendly reactions that have an effect on society, we want to understand how that wiring works. And so that’s what we’re making an attempt to determine,” he says.





:max_bytes(150000):strip_icc()/StephanieBrownnewheadshot-e5ca9ba2a404491384e9300a7871f190.jpg)







