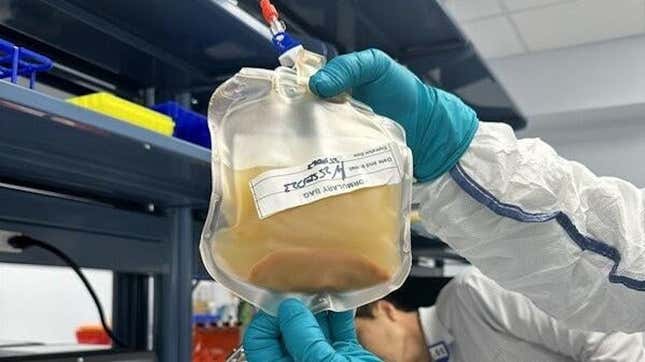
 LyGenesis cellular resolution with hepatocytes in suspension.Photograph: LyGenesisAn experimental liver-growing remedy is going through its largest check in people but. This week, biotech corporate LyGenesis introduced that it has simply begun a segment II trial of its treatment designed to develop miniature livers inside of an individual’s lymph nodes. If the treatment works as was hoping, it would probably save the lives of many of us with life-threatening liver illness who’re not able to acquire a standard transplant.
LyGenesis cellular resolution with hepatocytes in suspension.Photograph: LyGenesisAn experimental liver-growing remedy is going through its largest check in people but. This week, biotech corporate LyGenesis introduced that it has simply begun a segment II trial of its treatment designed to develop miniature livers inside of an individual’s lymph nodes. If the treatment works as was hoping, it would probably save the lives of many of us with life-threatening liver illness who’re not able to acquire a standard transplant.
Giancarlo Esposito on Having His Personal Motion FiguresThe remedy is lately known as LYG-LIV-001. It’s derived from donated livers that differently wouldn’t be a fit for any possible transplant recipient. Positive cells referred to as hepatocytes are gathered from those livers and suspended in an answer. The usage of a mix of minimally invasive surgical treatment and endoscopic ultrasound, the cells are then transplanted over to the recipient’s higher belly lymph nodes. From there, the lymph nodes are anticipated to behave as residing “bioreactors,” serving to the hepatocytes develop and mature into purposeful, if ectopic (outdoor of its same old place within the frame), liver tissue.LyGenesis is hoping to win acclaim for LYG-LIV-001 as a remedy for end-stage liver illness, or ESLD, a critical state of persistent organ harm. Even though any person can reside with ESLD for years, it’s estimated that about 2% of all deaths once a year are led to via it. Liver transplantation may also be an efficient remedy, however many with the situation don’t meet the standards for changing into an organ recipient and about 17% of other people at the ready checklist for a brand new liver die once a year. On Tuesday, LyGenesis reported that the primary ESLD affected person of its segment II trial has now won LYG-LIV-001.“This treatment will probably be a exceptional regenerative medication milestone via serving to sufferers with ESLD develop new purposeful ectopic livers in their very own frame,” stated LyGenesis CEO Michael Hufford in a observation. If our learn about is a hit and we download FDA approval, our allogenic cellular treatment may just permit one donated liver to regard many dozens of ESLD sufferers, which might assist to tilt the present organ supply-demand imbalance in desire of sufferers.”It is going to take months ahead of scientists can know whether or not the remedy has in reality helped this primary affected person, on the other hand. And the trial—set to sign up 12 sufferers in overall—isn’t anticipated to be finished till early 2026. But when this analysis assists in keeping appearing promise, then the sky could be the restrict. LyGenesis may be creating its bioreactor generation to develop different organs, together with the kidneys, thymus, and pancreas.
Experimental Remedy May just Develop You Logo New Livers














