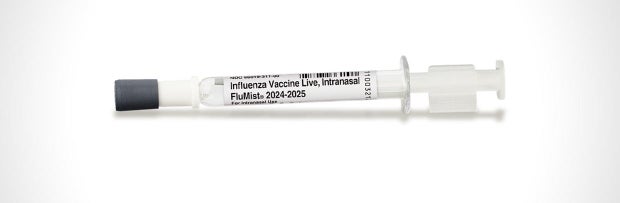
The Meals and Drug Management introduced Friday it had broadened the approval of the FluMist nasal spray to develop into the primary “self-administered” influenza vaccine — despite the fact that a prolong within the alternate method the vaccine may not be to be had to send to properties till subsequent 12 months’s flu season on the earliest.”These days’s approval of the primary influenza vaccine for self- or caregiver-administration supplies a brand new choice for receiving a protected and efficient seasonal influenza vaccine doubtlessly with larger comfort, flexibility and accessibility,” Dr. Peter Marks, director of the FDA’s Heart for Biologics Analysis and Analysis, stated in a commentary.The FluMist vaccine, manufactured by means of AstraZeneca, had in the past been authorized again in 2003 to be given by means of well being care suppliers very similar to different flu photographs. Now the vaccinemaker has approval to promote FluMist to adults to be used at house on themselves or to manage to their kids.
The FDA says sufferers will nonetheless wish to get a prescription for the vaccine from a physician. AstraZeneca says it plans to promote FluMist without delay to sufferers via a web based pharmacy. Adults will have the ability to whole a screening questionnaire to get the prescription, after which order shipments to their house.
There also are some limits to the sorts of folks FluMist is advisable for. Because it makes use of a are living however weakened model of the virus, some sufferers, like pregnant folks or those that are critically immunocompromised, must now not get this vaccine.
The FDA authorized AstraZeneca’s FluMist flu vaccine spray for self-administered use at house.
AstraZeneca
FluMist is much less recurrently used nowadays by means of pharmacies and docs, partially because of fallout from a Facilities for Illness Keep watch over and Prevention advice in 2016 towards use of the spray over “deficient or rather decrease effectiveness” in comparison to different vaccines.AstraZeneca later redesigned the antigens within the vaccine, incomes again the CDC’s advice beginning in 2018. Since then, the CDC says it has now not had sufficient knowledge for brand new professional effectiveness estimates evaluating FluMist to different flu vaccines, “as a result of restricted use” within the U.S.
However AstraZeneca has cited knowledge appearing the shot has had “similar” effectiveness in Europe as opposed to extra broadly used photographs.AstraZeneca had to begin with informed buyers it was hoping the FDA would increase approval in time for this flu season, after the corporate submitted knowledge final 12 months appearing that adults have been ready to as it should be observe directions to manage the vaccine spray on their very own.AstraZeneca didn’t remark when requested why the FDA’s approval determination got here later than the corporate in the past stated it anticipated.”We are running diligently to carry this ‘first-of-its-kind’ cutting edge and handy self-administrated flu vaccine to shoppers and stay up for launching FluMist House once subsequent flu season,” a spokesperson for the corporate stated in a commentary.The spokesperson stated AstraZeneca wanted time to paintings with its companions to “ensure that a unbroken buyer enjoy” for FluMist’s rollout for house use.
Extra
Alexander Tin
Alexander Tin is a virtual reporter for CBS Information based totally within the Washington, D.C. bureau. He covers the Biden management’s public well being businesses, together with the federal reaction to infectious illness outbreaks like COVID-19.