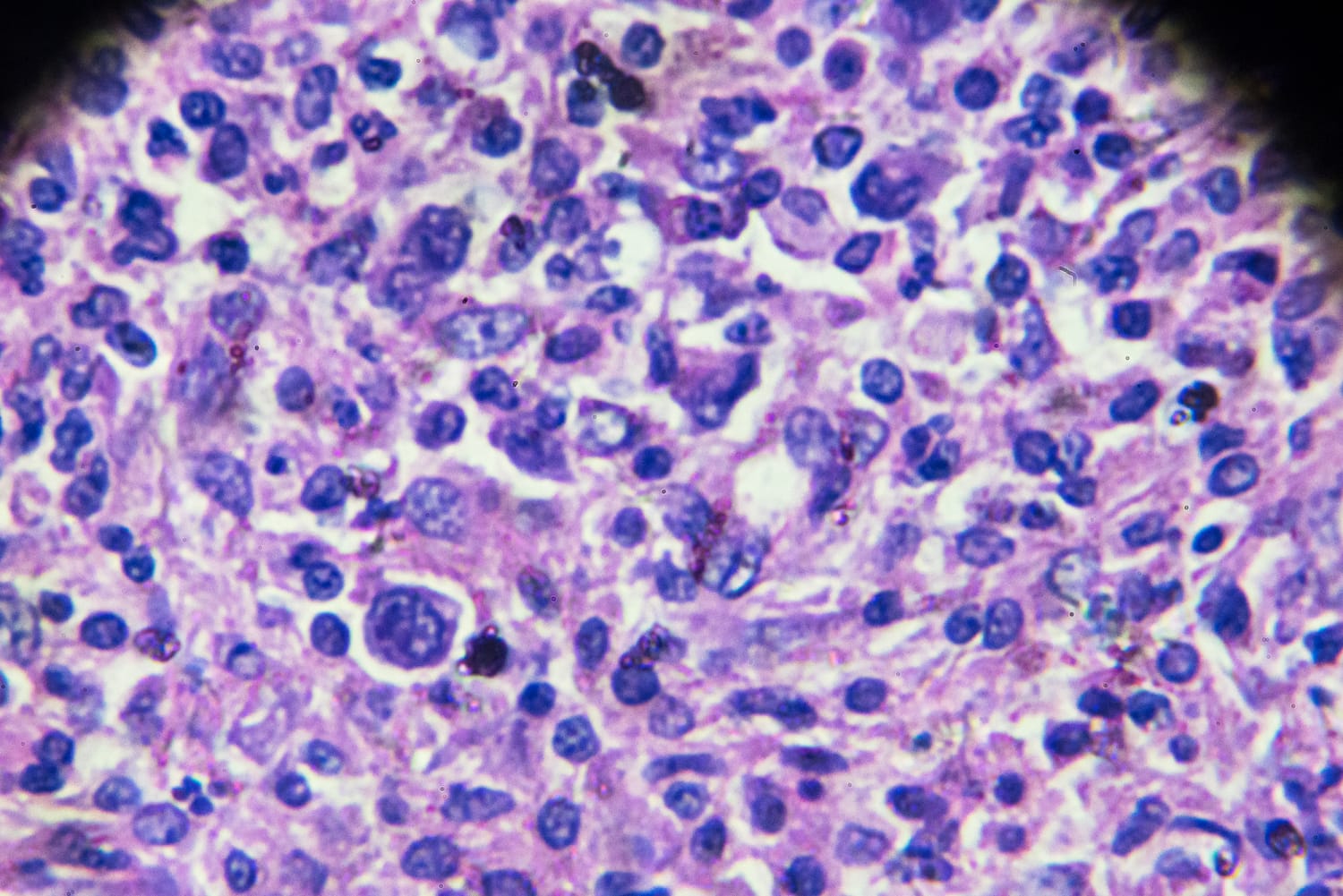
The Meals and Drug Management this week advised a number of drugmakers so as to add a boxed caution — the company’s most powerful protection label — to the prescribing knowledge for a kind of most cancers remedy known as CAR-T treatment, announcing the remedy itself might building up an individual’s chance of most cancers.Carly Kempler, a spokesperson for the FDA, mentioned that, in spite of the caution, “the full advantages of those merchandise proceed to outweigh their doable dangers.”The company’s choice to replace the labels was once in accordance with stories of uncommon blood cancers in sufferers who had in the past gotten CAR-T treatment, Kempler mentioned. As of Monday, the company had gained 25 stories of the blood cancers in CAR-T sufferers, she mentioned. Bruce Levine, a professor in most cancers gene treatment on the College of Pennsylvania, mentioned that along with the stories submitted to the FDA, two abstracts printed overdue ultimate yr within the magazine Blood additionally cited a possible most cancers chance related to CAR-T treatment, which most probably “compelled the FDA’s hand.”CAR-T — or chimeric antigen receptor T mobile — treatment makes use of a affected person’s personal immune cells to regard positive blood cancers, equivalent to leukemia, more than one myeloma and lymphoma. It comes to harvesting the immune cells — on this case, T cells — then genetically changing them in a lab to cause them to goal most cancers cells, and in the end reinfusing them again into the affected person.It’s confirmed to be extremely efficient in hard-to-treat instances, mavens mentioned. In 2022, docs who had handled two leukemia sufferers with CAR-T a decade in the past mentioned it was once truthful to mention the treatment had cured the sufferers of the illness.“This has been a recreation changer once we consider treating lymphoma and different sicknesses,” mentioned Dr. Matthew Frigault, the scientific director of the Massachusetts Common Health center Mobile Immunotherapy Program in Boston. The primary CAR-T treatment, Novartis’ drug Kymriah, gained FDA approval in 2017. Since then, any other 5 were authorized. The makers of 5 of the medication — Bristol Myers Squibb, for Abecma and Breyanzi; Gilead Sciences’ Kite Pharma, for Yescarta; Johnson & Johnson’s Carvykti; and Novartis, for Kymriah — gained letters from the FDA, declaring that they will have to put up proposed label adjustments within the subsequent 30 days to notice that, in uncommon instances, CAR-T treatment can building up the danger of uncommon blood cancers. (Kite Pharma didn’t obtain a letter in regards to the 6th CAR-T drug, Tecartus.)If the drugmakers disagree, they are able to as an alternative put up a rebuttal explaining why a transformation isn’t wanted.In a observation to NBC Information, a spokesperson for Novartis mentioned the corporate has no longer discovered “enough proof” to enhance a hyperlink between most cancers and its remedy, which has been utilized in greater than 10,000 sufferers. Alternatively, the spokesperson mentioned, the corporate will paintings with the FDA to replace its label “accurately.”Spokespersons for Johnson & Johnson and Gilead Sciences additionally mentioned the drugmakers would paintings with the company to replace their labels.A spokesperson for Bristol Myers Squibb mentioned the corporate is comparing “subsequent steps” following the FDA’s realize, despite the fact that it has no longer noticed any most cancers instances related to its remedy.“Affected person protection is our best precedence,” the spokesperson mentioned. How would possibly CAR-T treatment reason most cancers?Nonetheless, there’s the query of ways CAR-T may reason most cancers — if it does in any respect.“We in truth don’t know whether or not that is informal, which means, we don’t know for a proven fact that the CAR-T cells within the tumor have resulted in this,” mentioned Frigault, of Mass Common. RecommendedCAR-T therapies are nonetheless rather new: Frigault famous that the FDA has required that the makers of the goods behavior 15-year follow-up research to evaluate the possible chance of secondary cancers following remedy. (Secondary cancers are cancers that may rise up from remedy.) The FDA “isn’t announcing that each unmarried some of the instances they’ve reported has obviously proven CAR-T has resulted in this,” he mentioned, “however extra that there is also an affiliation.”“That is what the FDA does. They search for a sign,” he added.If CAR-T does reason most cancers, the danger is most probably very small, mentioned Dr. Hemant Murthy, a hematology-oncology doctor on the Mayo Health facility in Jacksonville, Florida.Greater than 27,000 doses of CAR-T treatment were administered within the U.S., in keeping with the FDA. “I don’t in point of fact see this affecting an excessive amount of of apply,” Murthy mentioned.Dr. Saad Usmani, a myeloma doctor and mobile therapist at Memorial Sloan Kettering, mentioned the label exchange will have to enhance physicians’ present apply of discussing with sufferers the danger of growing secondary cancers following most cancers remedy. Usmani famous that different most cancers therapies, equivalent to radiation and chemotherapy, additionally raise a chance of secondary cancers.“The exchange is predicted given the hot stories, albeit very low prevalence in such instances,” he mentioned. Dr. Marcela Maus, an affiliate professor of drugs at Harvard Clinical Faculty and director of the Mobile Immunotherapy Program at Massachusetts Common Health center, mentioned physicians may well be extra wary, however it perhaps received’t exchange a lot of their apply.“We want to organize the most cancers that they have got now, so I don’t believe it being vastly other,” she mentioned.Berkeley Lovelace Jr. is a well being and clinical reporter for NBC Information. He covers the Meals and Drug Management, with a unique center of attention on Covid vaccines, prescription drug pricing and well being care. He in the past lined the biotech and pharmaceutical trade with CNBC.
FDA says most cancers remedy CAR-T treatment might building up chance of most cancers