Sudafed, Mucinex, Benadryl, Advil, Tylenol, Vicks, and Dimetapp.Those standard emblem names seem on oral decongestants which might be staples of the chilly and flu aisle in American drug retail outlets, and but many include an element that doesn’t paintings as promised.
America Meals and Drug Management (FDA) has now proposed an order to take away oral phenylephrine from each and every unmarried chilly, cough, allergic reaction, bronchodilator, and anti-asthmatic drug product to be had these days, kind of four-fifths of all oral decongestants.
The proposal is now open for public remark, and, if finalized, the ruling would dramatically reshape the drug formulations noticed in loads of over the counter oral decongestants in the stores within the nation – a marketplace percentage price kind of US$1.76 billion in 2022.
In style merchandise impacted via the proposal would come with Advil Sinus Congestion & Ache, Sudafed PE Nasal Decongestant, Vicks DayQuil and NyQuil, and Tylenol Chilly & Flu Serious, simply to call a trifling few.
The proposed order comes a yr after an impartial advisory frame for the FDA unanimously concluded that whilst oral phenylephrine is protected to eat, it’s no higher than a placebo at clearing a stuffy nostril.
For just about twenty years now, some scientists have known as for the elimination of oral phenylephrine from the marketplace. The remaining time the FDA reviewed the drugs, then again, it saved the drug on cabinets.
“It’s the FDA’s position to make sure that medicine are protected and efficient,” says Patrizia Cavazzoni, director of the management’s Middle for Drug Analysis and Analysis (CDER).
“In line with our evaluate of to be had knowledge, and in keeping with the recommendation of the advisory committee, we’re taking this subsequent step within the procedure to suggest eliminating oral phenylephrine as a result of it isn’t efficient as a nasal decongestant.”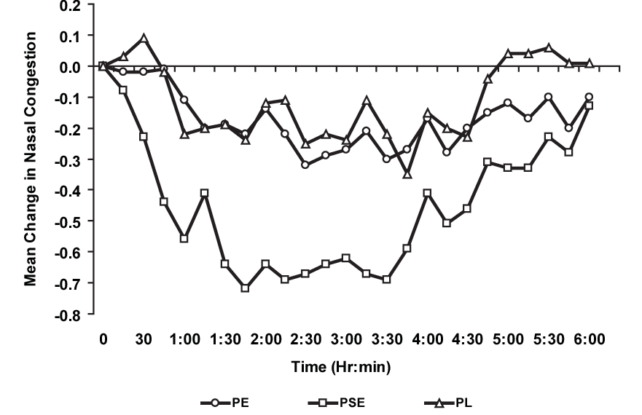 Imply alternate in subjective nasal congestion ratings at 15-minute periods after management of phenylephrine (PE), pseudoephedrine (PSE), and a placebo (PL). (Horak et al., Annals of Allergic reaction, Bronchial asthma & Immunology, 2009)To know the way such a lot of the drug marketplace got here to be ruled via a unnecessary decongestant, it is vital to appear again on the historical past of chilly and flu meds.
Imply alternate in subjective nasal congestion ratings at 15-minute periods after management of phenylephrine (PE), pseudoephedrine (PSE), and a placebo (PL). (Horak et al., Annals of Allergic reaction, Bronchial asthma & Immunology, 2009)To know the way such a lot of the drug marketplace got here to be ruled via a unnecessary decongestant, it is vital to appear again on the historical past of chilly and flu meds.
Phenylephrine used to be first authorized via the FDA as a protected and efficient decongestant in 1976, in response to most commonly industry-funded research that experience since been criticized for his or her technique.
Earlier than 2006, pseudoephedrine used to be the principle element in over the counter decongestants. Within the early 2000s, a federal regulation known as for states to have complete measures in position to keep watch over the drug’s sale because of considerations it used to be getting used within the manufacture of methamphetamine.
Since then, native regulations have both required a prescription for drugs containing pseudoephedrine, or have restricted quantities that may be bought from in the back of the counter.
After this ruling, over the counter decongestants in drug retail outlets, grocery retail outlets, and comfort retail outlets national had their pseudoephedrine changed with phenylephrine.
In 2005, some scientists reviewed current proof that confirmed phenylephrine used to be useless at de-clogging the nostril when taken orally on the prompt dosage.
In 2007, a citizen’s petition requested the FDA to require higher evidence of efficacy. On the time, then again, officers on the management known as for extra analysis on upper dosages.
Beginning in 2015, scientific trials attempted quadrupling the dosage of oral phenylephrine, however the medication nonetheless proved unnecessary as a decongestant, prompting every other citizen’s petition to take away those merchandise from the marketplace.
Now, after years of discussion, the company has been swayed via overwhelming proof. Final yr, the FDA committee analyzed 3 huge scientific trials that display oral phenylephrine isn’t efficient at any dose.
Research display that even if swallowed in upper doses, nearly no medication reaches the nasal passages. It’s most commonly damaged down within the intestine.
The proposed order to take away phenylephrine from oral decongestants does now not practice to nasal sprays or eye drops. Those merchandise ship the similar drug in some way this is more practical than an oral pill.
However maximum shoppers don’t seem to be conscious about the ones variations. In 2022, greater than 242 million chilly treatment merchandise containing phenylephrine had been bought in america – greater than 4 occasions as many as the ones containing pseudoephedrine.
Being a proposed order, the FDA is not requiring corporations to do anything else but. They’re, then again, on realize for additional motion, which can quickly require them to withdraw merchandise that include phenylephrine as the only real energetic element.A listing of oral decongestants containing phenylephrine may also be discovered right here.
FDA to After all Ban Debatable Aspect in In style Decongestants