From the spiral fingers of galaxies to microscopic snow crystals, nature turns out to fall into fractal-like patterns that repeat in increasingly more smaller increments. Regardless of how small you move, portions of the trend nonetheless resemble the entire.
One exception seems to be molecules, that have no longer been recognized to showcase self-similarity at converting scales. This is, till now.
Researchers from Germany, Sweden, and the United Kingdom have came upon an enzyme produced via a single-celled organism that may prepare itself right into a fractal – no longer simply any fractal, however a repeating trend of triangles referred to as a Sierpiński triangle.
The enzyme is a type of citrate synthase produced via the cyanobacterium Synechococcus elongatus, and its evolution from non-fractal precursors means that such molecular patterns can emerge unusually briefly.
“We stumbled in this construction utterly by chance and nearly could not consider what we noticed after we first took photographs of it the usage of an electron microscope,” says biochemist Franziska Sendker of the Max Planck Institute for Terrestrial Microbiology in Germany.
“The protein makes those stunning triangles and because the fractal grows, we see those better and bigger triangular voids in the course of them, which is completely in contrast to any protein meeting we have now ever noticed earlier than.” The Sierpiński triangle is without doubt one of the poster youngsters for fractal geometry. (Solkoll/Wikimedia Commons/Public Area)There is a reason why for that, the researchers discovered. They used electron microscopy to review the molecule’s construction at an atomic degree, inspecting how the protein chains are related.
The Sierpiński triangle is without doubt one of the poster youngsters for fractal geometry. (Solkoll/Wikimedia Commons/Public Area)There is a reason why for that, the researchers discovered. They used electron microscopy to review the molecule’s construction at an atomic degree, inspecting how the protein chains are related.
Maximum molecules have a extremely symmetrical construction, with every protein chain falling into the similar association and courting to the protein chains round it. This actual construction of citrate synthase manages to wreck that rule: the protein chains hyperlink up in relatively other ways, relying on their place within the molecule.
The result’s the Sierpiński association, and, curiously, the staff’s analysis discovered that it sort of feels to were a whole coincidence that has no serve as by any means. When the staff genetically manipulated S. elongatus to supply non-fractal citrate synthase, it made no distinction to the bacterium in any respect.
“Self-assembly is steadily utilized by evolution to control enzymes, however on this case the cyanobacterium that this enzyme is located in does no longer appear to care a lot whether or not or no longer its citrate synthase can collect right into a fractal,” says evolutionary biologist Georg Hochberg of the Max Planck Institute for Terrestrial Microbiology.
“This triggered us to wonder if this would possibly simply be a innocuous coincidence of evolution. Such injuries can occur when the construction in query is not too tough to build.”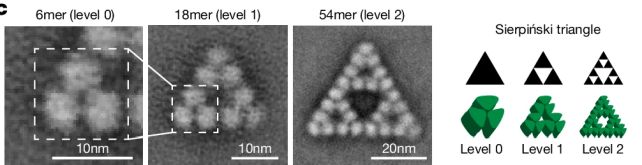 Electron microscopy photographs of the molecule at other ranges. (Sendker et al., Nature, 2024)And it sort of feels {that a} fractal trend is not an overly tough factor for a molecule to reach. The researchers explored the evolutionary historical past via the usage of ancestral series reconstruction to decide the precursors of the citrate synthase molecule as it sounds as if nowadays, and the amino acid substitutions that can have altered its construction through the years.
Electron microscopy photographs of the molecule at other ranges. (Sendker et al., Nature, 2024)And it sort of feels {that a} fractal trend is not an overly tough factor for a molecule to reach. The researchers explored the evolutionary historical past via the usage of ancestral series reconstruction to decide the precursors of the citrate synthase molecule as it sounds as if nowadays, and the amino acid substitutions that can have altered its construction through the years.
It seems that it takes just a very small selection of mutations to change the molecule’s form, with startling rapidity. However simple come is straightforward move – it may well disappear once more simply as briefly. Actually, the staff’s analysis means that fractal molecules have emerged and disappeared in several species of cyanobacteria a couple of instances prior to now.
It is simply that, if a fractal construction isn’t biologically useful, there is not any reason why for the organism to retain it, so off it is going. For S. elongatus, it is conceivable that the Sierpiński molecule is simplest brief. Or most likely in nature there may be some receive advantages that was once no longer seen in a laboratory atmosphere.
“We suspect that evolutionary transitions in self-assembly is also extra not unusual than structural databases make it appear,” the researchers write of their paper.
“Most likely just a small fraction ever turn into necessary to their organisms and persist. Many others will fade as briefly as they gave the impression. We will be able to subsequently simplest surprise about what number of distinctive assemblies have developed over the eons that by no means made it to the current for us to look at.”The findings were revealed in Nature.
First Fractal Molecule in Nature Assembles Right into a Sierpinski Triangle And We Don't Know Why