 In a contemporary medical trial, CRISPR gene enhancing used to be implemented to fourteen sufferers affected by a type of inherited blindness. The remedy proved protected and ended in measurable imaginative and prescient enhancements in 11 of the individuals. The trial, named BRILLIANCE, indicates an important step ahead in gene treatment for ocular illnesses. Credit score: SciTechDaily.comMass Eye and Ear-led section 1/2 trial, which integrated 14 individuals, discovered that the first-of-its-kind experimental remedy used to be protected and efficacious.BRILLIANCE trial effects confirmed 11 out of 14 handled individuals skilled some enhancements in imaginative and prescient and high quality of lifestyles measures.CRISPR-based treatment used to be discovered protected and not using a dose-limiting toxicities reported.Mass Eye and Ear researchers say their findings reinforce endured analysis and medical trials of CRISPR treatments for inherited retinal problems.Effects from a groundbreaking medical trial of CRISPR gene enhancing in 14 people with a type of inherited blindness display that the remedy is protected and ended in measurable enhancements in 11 of the individuals handled. The section 1/2 trial known as BRILLIANCE, used to be led by way of main investigator Eric Pierce, MD, PhD, of Mass Eye and Ear, a member of the Mass Normal Brigham healthcare gadget, and backed by way of Editas Medication, Inc. Findings are reported on Might sixth in The New England Magazine of Medication.“This analysis demonstrates that CRISPR gene treatment for inherited imaginative and prescient loss is price endured pursuit in analysis and medical trials,” mentioned Pierce, director of Ocular Genomics Institute and Berman-Gund Laboratory for the Learn about of Retinal Degenerations at Mass Eye and Ear and Harvard Scientific College. “Whilst extra analysis is had to resolve who could gain advantage maximum, we believe the early effects promising. To listen to from a number of individuals how extremely joyful they had been that they may in the end see the meals on their plates –that may be a large deal. Those had been people who may now not learn any traces on an eye fixed chart and who had no remedy choices, which is the unlucky fact for the general public with inherited retinal problems.”
In a contemporary medical trial, CRISPR gene enhancing used to be implemented to fourteen sufferers affected by a type of inherited blindness. The remedy proved protected and ended in measurable imaginative and prescient enhancements in 11 of the individuals. The trial, named BRILLIANCE, indicates an important step ahead in gene treatment for ocular illnesses. Credit score: SciTechDaily.comMass Eye and Ear-led section 1/2 trial, which integrated 14 individuals, discovered that the first-of-its-kind experimental remedy used to be protected and efficacious.BRILLIANCE trial effects confirmed 11 out of 14 handled individuals skilled some enhancements in imaginative and prescient and high quality of lifestyles measures.CRISPR-based treatment used to be discovered protected and not using a dose-limiting toxicities reported.Mass Eye and Ear researchers say their findings reinforce endured analysis and medical trials of CRISPR treatments for inherited retinal problems.Effects from a groundbreaking medical trial of CRISPR gene enhancing in 14 people with a type of inherited blindness display that the remedy is protected and ended in measurable enhancements in 11 of the individuals handled. The section 1/2 trial known as BRILLIANCE, used to be led by way of main investigator Eric Pierce, MD, PhD, of Mass Eye and Ear, a member of the Mass Normal Brigham healthcare gadget, and backed by way of Editas Medication, Inc. Findings are reported on Might sixth in The New England Magazine of Medication.“This analysis demonstrates that CRISPR gene treatment for inherited imaginative and prescient loss is price endured pursuit in analysis and medical trials,” mentioned Pierce, director of Ocular Genomics Institute and Berman-Gund Laboratory for the Learn about of Retinal Degenerations at Mass Eye and Ear and Harvard Scientific College. “Whilst extra analysis is had to resolve who could gain advantage maximum, we believe the early effects promising. To listen to from a number of individuals how extremely joyful they had been that they may in the end see the meals on their plates –that may be a large deal. Those had been people who may now not learn any traces on an eye fixed chart and who had no remedy choices, which is the unlucky fact for the general public with inherited retinal problems.”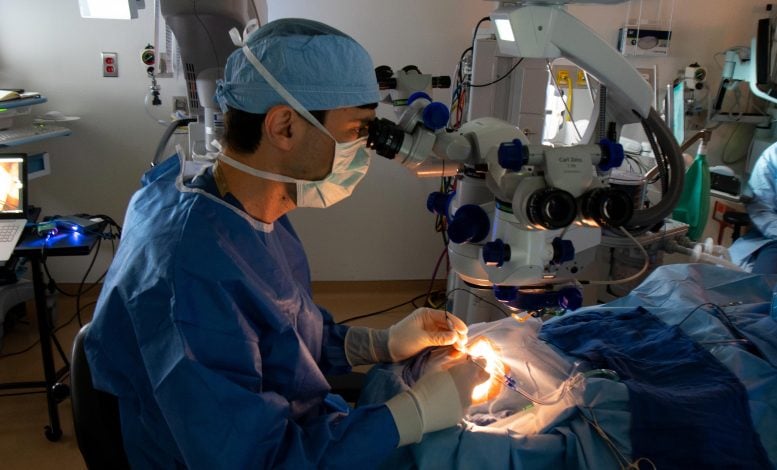 Jason Comander, MD, PhD, plays the process to ship the CRISPR-based drugs as a part of the BRILLIANCE trial in September 2020 at Mass Eye and Ear. Credit score: Mass Eye and EarParticipant Demographics and Trial ProceduresAll 14 trial individuals, together with 12 adults (ages 17 to 63) and two kids (ages 10 and 14), had been born with a type of Leber Congenital Amaurosis (LCA) brought about by way of mutations within the centrosomal protein 290 (CEP290) gene. They underwent a unmarried injection of a CRISPR/Cas9 genome enhancing drugs, EDIT-101 in a single eye by means of a specialised surgical process. This trial, which integrated the 1st affected person to ever obtain a CRISPR-based investigational drugs at once throughout the frame, centered totally on protection with a secondary research for efficacy.No critical remedy or procedure-related opposed occasions had been reported, nor had been there any dose-limiting toxicities. For efficacy, the researchers checked out 4 measures: best-corrected visible acuity (BCVA); dark-adapted full-field stimulus checking out (FST), visible serve as navigation (VNC, as measured by way of a maze individuals finished), and vision-related high quality of lifestyles.11 individuals demonstrated enhancements in a minimum of a kind of results, whilst six demonstrated growth in two or extra. 4 individuals had clinically significant growth in BCVA. Six individuals skilled significant enhancements in cone-mediated imaginative and prescient as indicated by way of FSTs, 5 of whom had enhancements in a minimum of some of the 3 different results. Cone photoreceptors are used for daylight and central imaginative and prescient.
Jason Comander, MD, PhD, plays the process to ship the CRISPR-based drugs as a part of the BRILLIANCE trial in September 2020 at Mass Eye and Ear. Credit score: Mass Eye and EarParticipant Demographics and Trial ProceduresAll 14 trial individuals, together with 12 adults (ages 17 to 63) and two kids (ages 10 and 14), had been born with a type of Leber Congenital Amaurosis (LCA) brought about by way of mutations within the centrosomal protein 290 (CEP290) gene. They underwent a unmarried injection of a CRISPR/Cas9 genome enhancing drugs, EDIT-101 in a single eye by means of a specialised surgical process. This trial, which integrated the 1st affected person to ever obtain a CRISPR-based investigational drugs at once throughout the frame, centered totally on protection with a secondary research for efficacy.No critical remedy or procedure-related opposed occasions had been reported, nor had been there any dose-limiting toxicities. For efficacy, the researchers checked out 4 measures: best-corrected visible acuity (BCVA); dark-adapted full-field stimulus checking out (FST), visible serve as navigation (VNC, as measured by way of a maze individuals finished), and vision-related high quality of lifestyles.11 individuals demonstrated enhancements in a minimum of a kind of results, whilst six demonstrated growth in two or extra. 4 individuals had clinically significant growth in BCVA. Six individuals skilled significant enhancements in cone-mediated imaginative and prescient as indicated by way of FSTs, 5 of whom had enhancements in a minimum of some of the 3 different results. Cone photoreceptors are used for daylight and central imaginative and prescient. Infographic explaining the section 1/2 result of the BRILLIANCE trial. Credit score: Mass Normal BrighamCRISPR’s Attainable and Early Successes“The effects from the BRILLIANCE trial supply evidence of thought and necessary learnings for the advance of recent and cutting edge drugs for inherited retinal illnesses. We’ve demonstrated that we will safely ship a CRISPR-based gene enhancing healing to the retina and feature clinically significant results,” mentioned Baisong Mei, MD, PhD, Leader Scientific Officer, Editas Medication.Research like this one display the promise of gene treatment for treating incurable prerequisites. Mass Normal Brigham’s Gene and Mobile Treatment Institute helps to translate clinical discoveries made by way of researchers into first-in-human medical trials and, in the end, life-changing therapies for sufferers.Mutations within the CEP290 gene are the main reason for inherited blindness going down throughout the 1st decade of lifestyles. The mutations purpose rod and cone photoceptors within the eye’s retina to serve as improperly, which after a while will result in irreversible imaginative and prescient loss. Pierce compares it to a small a part of an engine breaking down, which ultimately leads all the engine to falter.CRISPR-Cas9 is a gene enhancing toolkit that acts as a GPS-guided scissor to chop a portion of the mutated genome to depart a practical gene. For inherited blindness, the function used to be to inject CRISPR to succeed in the attention’s retina to revive the power to provide the gene and protein chargeable for light-sensing cells.The CEP290 gene is bigger than what conventional adeno-associated virus (AAV) vector gene treatments, together with one FDA-approved for a unique form of inherited imaginative and prescient loss, can accommodate. The genome enhancing corporate Editas Medication started exploring how you can take on the CEP290 mutation in 2014, engaging in preclinical research to resolve whether or not a gene enhancing means like CRISPR-Cas9 could be possible to focus on those massive gene mutations. This paintings ended in the BRILLIANCE trial, which started in mid-2019.
Infographic explaining the section 1/2 result of the BRILLIANCE trial. Credit score: Mass Normal BrighamCRISPR’s Attainable and Early Successes“The effects from the BRILLIANCE trial supply evidence of thought and necessary learnings for the advance of recent and cutting edge drugs for inherited retinal illnesses. We’ve demonstrated that we will safely ship a CRISPR-based gene enhancing healing to the retina and feature clinically significant results,” mentioned Baisong Mei, MD, PhD, Leader Scientific Officer, Editas Medication.Research like this one display the promise of gene treatment for treating incurable prerequisites. Mass Normal Brigham’s Gene and Mobile Treatment Institute helps to translate clinical discoveries made by way of researchers into first-in-human medical trials and, in the end, life-changing therapies for sufferers.Mutations within the CEP290 gene are the main reason for inherited blindness going down throughout the 1st decade of lifestyles. The mutations purpose rod and cone photoceptors within the eye’s retina to serve as improperly, which after a while will result in irreversible imaginative and prescient loss. Pierce compares it to a small a part of an engine breaking down, which ultimately leads all the engine to falter.CRISPR-Cas9 is a gene enhancing toolkit that acts as a GPS-guided scissor to chop a portion of the mutated genome to depart a practical gene. For inherited blindness, the function used to be to inject CRISPR to succeed in the attention’s retina to revive the power to provide the gene and protein chargeable for light-sensing cells.The CEP290 gene is bigger than what conventional adeno-associated virus (AAV) vector gene treatments, together with one FDA-approved for a unique form of inherited imaginative and prescient loss, can accommodate. The genome enhancing corporate Editas Medication started exploring how you can take on the CEP290 mutation in 2014, engaging in preclinical research to resolve whether or not a gene enhancing means like CRISPR-Cas9 could be possible to focus on those massive gene mutations. This paintings ended in the BRILLIANCE trial, which started in mid-2019.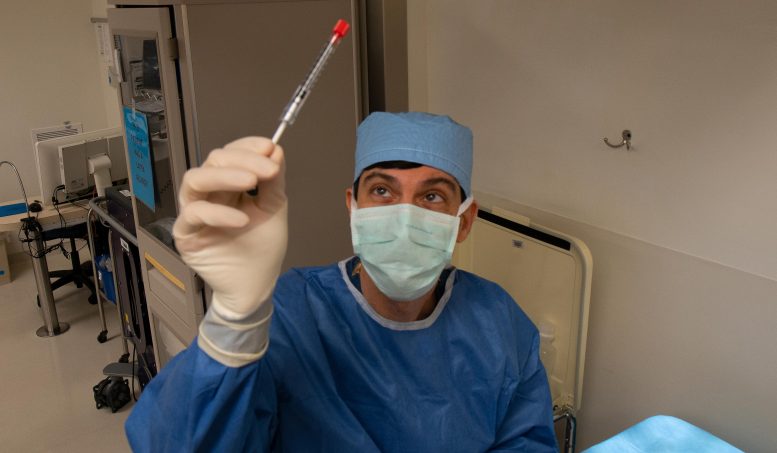 Jason Comander, MD, PhD, director of the Inherited Retinal Problems Provider at Mass Eye and Ear, examines the CRISPR-based drugs previous to appearing a surgical treatment of the radical remedy in September 2020, at Mass Eye and Ear in Boston. Credit score: Mass Eye and EarTrial Results and Long term DirectionsThe first affected person to obtain a CRISPR remedy throughout the frame (in vivo) came about on the Casey Eye Institute at Oregon Well being & Science College (OHSU), underneath the management of Mark Pennesi, MD, PhD.“This trial displays CRISPR gene enhancing has thrilling possible to regard inherited retinal degeneration,” Pennesi mentioned. “There may be not anything extra rewarding to a doctor than listening to a affected person describe how their imaginative and prescient has progressed after a remedy. One among our trial individuals has shared a number of examples, together with with the ability to in finding their telephone after misplacing it and figuring out that their espresso gadget is operating by way of seeing its small lighting. Whilst a lot of these duties may appear trivial to those that are most often sighted, such enhancements will have an enormous have an effect on on high quality of lifestyles for the ones with low imaginative and prescient.”The second one affected person used to be handled at Mass Eye and Ear in September 2020, following delays brought about by way of the COVID-19 pandemic. Further individuals had been handled throughout 3 different trial websites: Bascom Palmer Eye Institute, W.Ok. Kellogg Eye Middle, and Scheie Eye Institute on the Youngsters’s Sanatorium of Philadelphia (CHOP) and the Sanatorium of the College of Pennsylvania. Two adults won low-dose treatment, 5 won mid-dose, and some other 5 won a high-dose remedy. Two kids, handled at CHOP underneath the management of Tomas S. Aleman, MD, won a mid-dose remedy.
Jason Comander, MD, PhD, director of the Inherited Retinal Problems Provider at Mass Eye and Ear, examines the CRISPR-based drugs previous to appearing a surgical treatment of the radical remedy in September 2020, at Mass Eye and Ear in Boston. Credit score: Mass Eye and EarTrial Results and Long term DirectionsThe first affected person to obtain a CRISPR remedy throughout the frame (in vivo) came about on the Casey Eye Institute at Oregon Well being & Science College (OHSU), underneath the management of Mark Pennesi, MD, PhD.“This trial displays CRISPR gene enhancing has thrilling possible to regard inherited retinal degeneration,” Pennesi mentioned. “There may be not anything extra rewarding to a doctor than listening to a affected person describe how their imaginative and prescient has progressed after a remedy. One among our trial individuals has shared a number of examples, together with with the ability to in finding their telephone after misplacing it and figuring out that their espresso gadget is operating by way of seeing its small lighting. Whilst a lot of these duties may appear trivial to those that are most often sighted, such enhancements will have an enormous have an effect on on high quality of lifestyles for the ones with low imaginative and prescient.”The second one affected person used to be handled at Mass Eye and Ear in September 2020, following delays brought about by way of the COVID-19 pandemic. Further individuals had been handled throughout 3 different trial websites: Bascom Palmer Eye Institute, W.Ok. Kellogg Eye Middle, and Scheie Eye Institute on the Youngsters’s Sanatorium of Philadelphia (CHOP) and the Sanatorium of the College of Pennsylvania. Two adults won low-dose treatment, 5 won mid-dose, and some other 5 won a high-dose remedy. Two kids, handled at CHOP underneath the management of Tomas S. Aleman, MD, won a mid-dose remedy. Most important investigator of the BRILLIANCE trial, Eric Pierce, MD, PhD, director of Ocular Genomics Institute and Berman-Gund Laboratory for the Learn about of Retinal Degenerations at Mass Eye and Ear and Harvard Scientific College. Credit score: Mass Eye and Ear“Our sufferers are the 1st congenitally blind kids to be handled with gene-editing, which considerably progressed their daylight imaginative and prescient. Our hope is that the find out about will pave the street for therapies of more youthful kids with equivalent prerequisites and extra enhancements in imaginative and prescient,” mentioned Aleman, the Irene Heinz-Given and John LaPorte Analysis Professor in Ophthalmology at Penn Medication with the Scheie Eye Institute and a pediatric ophthalmologist at CHOP who served as a web page main investigator and find out about co-author. “This trial represents a landmark within the remedy of genetic illnesses, in particular, genetic blindness, by way of providing the most important choice remedy, when conventional sorts of gene treatment, similar to gene augmentation, don’t seem to be an choice.”Individuals had been monitored each 3 months for 12 months, after which adopted much less steadily for 2 further years. At visits, they might go through a sequence of serum and imaginative and prescient assessments to inspect protection and efficacy result measures.In November 2022, Editas paused enrollment at the BRILLIANCE trial. Pierce and associates are exploring operating with different business companions to habits further trials, in collaboration with Editas. The researchers hope long term research can read about splendid dosing, whether or not a remedy impact is extra pronounced in positive age teams similar to more youthful sufferers, and come with subtle endpoints to measure the consequences of progressed cone serve as on actions of day-to-day residing.For extra in this analysis, see Pioneering CRISPR Gene Enhancing Trial: 79% of Individuals See Development.Reference: “Gene-editing for CEP290-associated Retinal Degeneration” by way of Eric A. Pierce, Tomas S. Aleman, Kanishka T. Jayasundera, Vibrant S. Ashimatey, Keunpyo Kim, Alia Rashid, Michael Jaskolka, Rene L. Myers, Bryon L. Lam, Steven T. Bailey, Jason I. Commander, Andreas Ok. Lauer, Albert M. Maguire and Mark E. Pennesi, 6 Might 2024, New England Magazine of Medication.
Most important investigator of the BRILLIANCE trial, Eric Pierce, MD, PhD, director of Ocular Genomics Institute and Berman-Gund Laboratory for the Learn about of Retinal Degenerations at Mass Eye and Ear and Harvard Scientific College. Credit score: Mass Eye and Ear“Our sufferers are the 1st congenitally blind kids to be handled with gene-editing, which considerably progressed their daylight imaginative and prescient. Our hope is that the find out about will pave the street for therapies of more youthful kids with equivalent prerequisites and extra enhancements in imaginative and prescient,” mentioned Aleman, the Irene Heinz-Given and John LaPorte Analysis Professor in Ophthalmology at Penn Medication with the Scheie Eye Institute and a pediatric ophthalmologist at CHOP who served as a web page main investigator and find out about co-author. “This trial represents a landmark within the remedy of genetic illnesses, in particular, genetic blindness, by way of providing the most important choice remedy, when conventional sorts of gene treatment, similar to gene augmentation, don’t seem to be an choice.”Individuals had been monitored each 3 months for 12 months, after which adopted much less steadily for 2 further years. At visits, they might go through a sequence of serum and imaginative and prescient assessments to inspect protection and efficacy result measures.In November 2022, Editas paused enrollment at the BRILLIANCE trial. Pierce and associates are exploring operating with different business companions to habits further trials, in collaboration with Editas. The researchers hope long term research can read about splendid dosing, whether or not a remedy impact is extra pronounced in positive age teams similar to more youthful sufferers, and come with subtle endpoints to measure the consequences of progressed cone serve as on actions of day-to-day residing.For extra in this analysis, see Pioneering CRISPR Gene Enhancing Trial: 79% of Individuals See Development.Reference: “Gene-editing for CEP290-associated Retinal Degeneration” by way of Eric A. Pierce, Tomas S. Aleman, Kanishka T. Jayasundera, Vibrant S. Ashimatey, Keunpyo Kim, Alia Rashid, Michael Jaskolka, Rene L. Myers, Bryon L. Lam, Steven T. Bailey, Jason I. Commander, Andreas Ok. Lauer, Albert M. Maguire and Mark E. Pennesi, 6 Might 2024, New England Magazine of Medication.
DOI: 10.1056/NEJMoa2309915The senior corresponding writer of this find out about used to be Eric A. Pierce, MD, PhD (Mass Eye and Ear), and Tomas S. Aleman, MD (CHOP) and Mark E. Pennesi, MD, PhD (OHSU) had been co-corresponding authors. Further co-authors come with Kanishka T. Jayasundera, MD (Kellogg), Vibrant S. Ashimatey, OD, PhD (Editas), Keunpyo Kim, PhD (Editas), Alia Rashid, MD (Editas), Michael C. Jaskolka, PhD (Editas), Rene L. Myers, PhD (Editas), Byron L. Lam, MD (Bascom Palmer), Steven T. Bailey, MD (OHSU), Jason I. Comander, MD, PhD (Mass Eye and Ear), Andreas Ok. Lauer, MD (OHSU), Albert M. Maguire, MD (CHOP).This analysis used to be funded by way of Editas drugs. This analysis used to be additionally supported by way of the Nationwide Institute of Well being P30 EY014104 core grant to Mass Eye and Ear, P30 EY010572 core grant, the Malcolm M. Marquis MD Endowed Fund for Innovation, and an unrestricted grants from Analysis to Save you Blindness to Casey Eye Institute and the Scheie Eye Institute. Further reinforce used to be equipped by way of the Irene Heinz Given and John L. a. Porte Given Endowment, and Hope for Imaginative and prescient.
Gene Enhancing Step forward: CRISPR Improves Imaginative and prescient in Scientific Trial












:max_bytes(150000):strip_icc()/MSTRChart.py-2c9306aabdaf4aa0bc6e0d6569996c7c.jpg)