Abstract: A lipid anchor on nerve mobile membranes stabilizes prion proteins (PrPC) and forestalls their pathological aggregation into paperwork related to prion sicknesses. Researchers evolved new in vitro and mobile fashions to check how membrane anchoring inhibits those damaging transformations.They discovered that whilst anchoring stabilizes prion proteins, pre-formed aggregates can nonetheless induce clumping, suggesting a mechanism related to infectious prion sicknesses. Those findings supply crucial insights into protein misfolding and would possibly tell healing methods for prion and different neurodegenerative sicknesses.Key Details:Lipid Anchors Position: Membrane anchors stabilize prion proteins, combating pathological aggregation.Illness Mechanism: Pre-formed aggregates can induce clumping of anchored prion proteins, related to infectious prion sicknesses.Healing Perception: Working out protein stabilization may just help in growing remedies for prion and comparable sicknesses.Supply: RUBProtein aggregation is standard of more than a few neurodegenerative sicknesses akin to Alzheimer’s, Parkinson’s and prion sicknesses akin to Creutzfeld-Jakob illness. A analysis crew headed by way of Professor Jörg Tatzelt from the Division of Biochemistry of Neurodegenerative Sicknesses at Ruhr College Bochum, Germany, has now used new in vitro and mobile tradition fashions to turn {that a} lipid anchor at the outer membrane of nerve cells inhibits the aggregation of the prion protein. 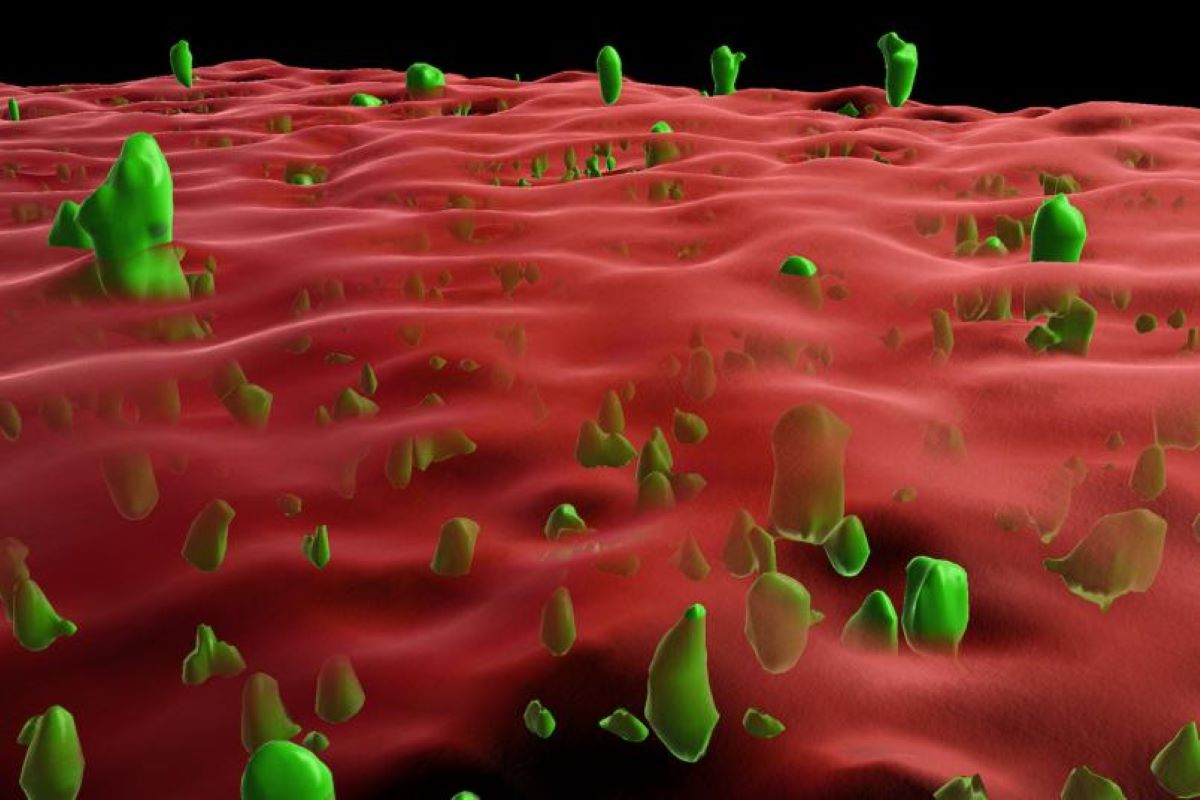 3-d reconstruction of a microscope symbol: crimson is the membrane and inexperienced is clumped prion protein. Credit score: AG Tatzelt“Working out the mechanisms that reason the at the start folded proteins to turn out to be into pathogenic paperwork is of a very powerful significance for the improvement of healing methods,” says Jörg Tatzelt.The crew revealed their findings within the magazine Complaints of the Nationwide Academy of Sciences (PNAS) on December, 31, 2024.Hereditary and infectious varieties of the diseasePrion sicknesses are deadly degenerative sicknesses of the mind. They’re related to the transformation of the cell prion protein (PrPC) from its wholesome fold into pathological aggregates, i.e. scrapie prion protein (PrPSc). Whilst such sicknesses are uncommon in people, hereditary prion sicknesses are prompted by way of genetic mutations.Some gene mutations impact the anchoring of PrPC to the mobile membrane. Alternatively, it’s nonetheless no longer totally understood precisely how those adjustments can cause prion sicknesses.With a purpose to acquire new insights into the underlying processes, the researchers have evolved new fashions to discover the function of a membrane anchor at the folding and aggregation of PrP in vitro and in neuronal cells.The experiments confirmed that anchoring to membranes stabilizes the folding of PrP and successfully inhibits aggregation.“What’s attention-grabbing is that the clumping of membrane-anchored PrP might be caused by way of pre-formed protein aggregates,” says Jörg Tatzelt.“It is a mechanism that would possibly play a job in infectious prion sicknesses.”Investment: The find out about used to be funded by way of the German Analysis Basis: TA 167/6-3, WI/2111-6 and Cluster of Excellence Ruhr Explores Solvation (RESOLV, EXC 2033–390677874).About this neurology and genetics analysis newsAuthor: Jörg Tatzelt
3-d reconstruction of a microscope symbol: crimson is the membrane and inexperienced is clumped prion protein. Credit score: AG Tatzelt“Working out the mechanisms that reason the at the start folded proteins to turn out to be into pathogenic paperwork is of a very powerful significance for the improvement of healing methods,” says Jörg Tatzelt.The crew revealed their findings within the magazine Complaints of the Nationwide Academy of Sciences (PNAS) on December, 31, 2024.Hereditary and infectious varieties of the diseasePrion sicknesses are deadly degenerative sicknesses of the mind. They’re related to the transformation of the cell prion protein (PrPC) from its wholesome fold into pathological aggregates, i.e. scrapie prion protein (PrPSc). Whilst such sicknesses are uncommon in people, hereditary prion sicknesses are prompted by way of genetic mutations.Some gene mutations impact the anchoring of PrPC to the mobile membrane. Alternatively, it’s nonetheless no longer totally understood precisely how those adjustments can cause prion sicknesses.With a purpose to acquire new insights into the underlying processes, the researchers have evolved new fashions to discover the function of a membrane anchor at the folding and aggregation of PrP in vitro and in neuronal cells.The experiments confirmed that anchoring to membranes stabilizes the folding of PrP and successfully inhibits aggregation.“What’s attention-grabbing is that the clumping of membrane-anchored PrP might be caused by way of pre-formed protein aggregates,” says Jörg Tatzelt.“It is a mechanism that would possibly play a job in infectious prion sicknesses.”Investment: The find out about used to be funded by way of the German Analysis Basis: TA 167/6-3, WI/2111-6 and Cluster of Excellence Ruhr Explores Solvation (RESOLV, EXC 2033–390677874).About this neurology and genetics analysis newsAuthor: Jörg Tatzelt
Supply: RUB
Touch: Jörg Tatzelt – RUB
Symbol: The picture is credited to AG TatzeltOriginal Analysis: Closed get right of entry to.
“Topological Confinement by way of a Membrane Anchor Suppresses Section Separation into Protein Aggregates: Implications for Prion Sicknesses” by way of Jörg Tatzelt et al. PNASAbstractTopological Confinement by way of a Membrane Anchor Suppresses Section Separation into Protein Aggregates: Implications for Prion DiseasesProtein misfolding and aggregation are a trademark of more than a few neurodegenerative problems. Alternatively, the underlying mechanisms using protein misfolding within the cell context are incompletely understood.Right here, we display that the two-dimensional confinement imposed by way of a membrane anchor stabilizes the local protein conformation and suppresses liquid–liquid segment separation (LLPS) and protein aggregation.Inherited prion sicknesses in people and neurodegeneration in transgenic mice are related to the expression of anchorless prion protein (PrP), suggesting that the C-terminal glycosylphosphatidylinositol (GPI) anchor of local PrP impedes spontaneous formation of neurotoxic and infectious PrP species.Combining distinctive in vitro and in vivo approaches, we display that anchoring to membranes prevents LLPS and spontaneous aggregation of PrP. Upon liberate from the membrane, PrP undergoes a conformational transition to detergent-insoluble aggregates.Our find out about demonstrates an crucial function of the GPI anchor in combating spontaneous misfolding of PrPC and gives a mechanistic foundation for inherited prion sicknesses related to anchorless PrP.
Lipid Anchors Hang Key to Combating Protein Aggregation in Prion Sicknesses – Neuroscience Information









![Equipment maker appearing Nintendo Transfer 2 dummy copy at CES 2025 [update: April launch claim] Equipment maker appearing Nintendo Transfer 2 dummy copy at CES 2025 [update: April launch claim]](https://nintendoeverything.com/wp-content/uploads/Genki-Nintendo-Switch-2-CES-2024-656x492.webp)




