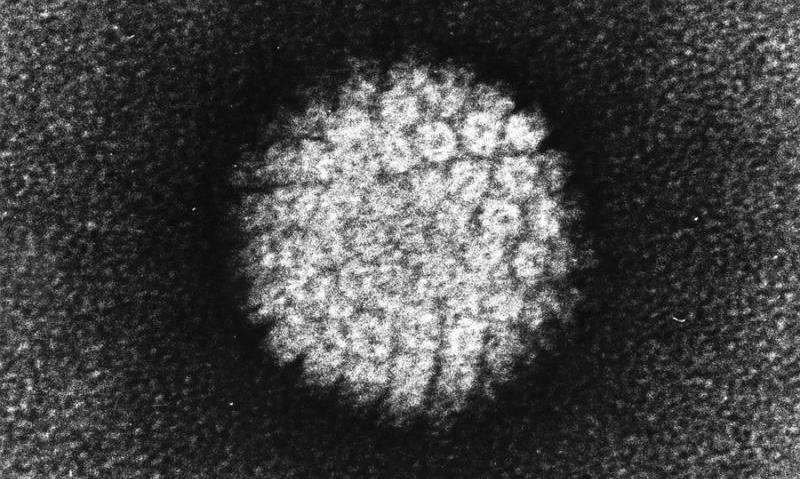
In an ongoing medical trial, researchers are checking out whether or not only one dose of a brand new gene treatment that would possibly successfully remedy human immunodeficiency virus (HIV) infections is secure in people. The treatment, named EBT-101, comes to the use of CRISPR-Cas9 gene enhancing to regard HIV. This possible remedy technique has been studied in animal fashions because the building of CRISPR-Cas9 in 2012. Then again, that is the primary time one of these gene-editing remedy for HIV has been attempted in people. The newest knowledge from the trial counsel that EBT-101 is secure on the doses examined, however we do not but know if it treatments HIV.In line with the United Countries Programme on HIV/AIDS (UNAIDS), roughly 39 million other folks globally have been dwelling with HIV in 2022, and there have been about 630,000 AIDS-related deaths that yr, making HIV a persevered public well being burden. There’s no vaccine or simply available remedy for HIV, even supposing a handful of other folks had been successfully cured thru in depth stem-cell transplants. The EBT-101 trial “is crucial step ahead within the building of this era to regard human illness and an infection, together with HIV,” Thomas Hope, a professor of cellular and developmental biology at Northwestern College who used to be no longer concerned within the paintings, advised Are living Science in an e mail. However how most probably is it, in reality, that shall we use CRISPR to remedy HIV sooner or later?Similar: Lets finish the AIDS epidemic in lower than a decade. Here is how.How CRISPR may (theoretically) remedy HIVHIV infects immune cells which can be typically used to combat an infection within the virus’s host. The virus makes use of an immune cellular’s equipment to insert its personal DNA into the host’s genome, permitting the virus to duplicate. If an HIV an infection isn’t handled, it can result in obtained immunodeficiency syndrome (AIDS), which ends up in a significantly weakened immune machine and leaves the inflamed individual extremely liable to different infections, cancers and early dying.Mixture antiretroviral treatments (cARTs) are the mainstay in HIV remedy and restrict the virus’ replication, thus extending other folks’s lives to near-normal lengths and slicing their probability of spreading HIV. Then again, those treatments will have to be taken for an entire life and fall in need of being a remedy.”The present problem in HIV remedy lies within the virus developing resilient genetic reservoirs inside human cells,” Elena Herrera-Carrillo, an affiliate professor of infectious illness at Amsterdam College Scientific Facilities, advised Are living Science. Herrera-Carrillo’s lab makes a speciality of the use of CRISPR to edit cells that harbor HIV reservoirs, in spite of ongoing cART treatment. This phenomenon is referred to as “latent HIV” and happens when the virus infects a kind of immune cellular referred to as CD4+ reminiscence T cells, which will persist for many years.cART treatments can suppress viral replication, but when the remedy is interrupted, “the dormant proviruses can reactivate, creating a remedy elusive,” Herrera-Carrillo advised Are living Science.CRISPR works through concentrated on and cleaving explicit sequences of DNA from the genome; a “information” leads CRISPR’s well-known “molecular scissors” to the centered gene. This both deactivates the gene or lets in it to be got rid of and swapped for various DNA. Analysis teams imagine this technique might be efficient in taking away latent HIV infections, as a result of it may goal the viral DNA embedded within the genome, quite than best preventing replication.The usage of CRISPR for HIV gene treatment has proven promise in numerous test-tube research, a 2020 overview in The Magazine of Scientific Investigation notes. More than a few teams had been operating to carry the treatments from check tubes to human sufferers — and that brings us to EBT-101.Similar: The sector’s 1st CRISPR treatment has simply been licensed. Here is the whole thing you want to grasp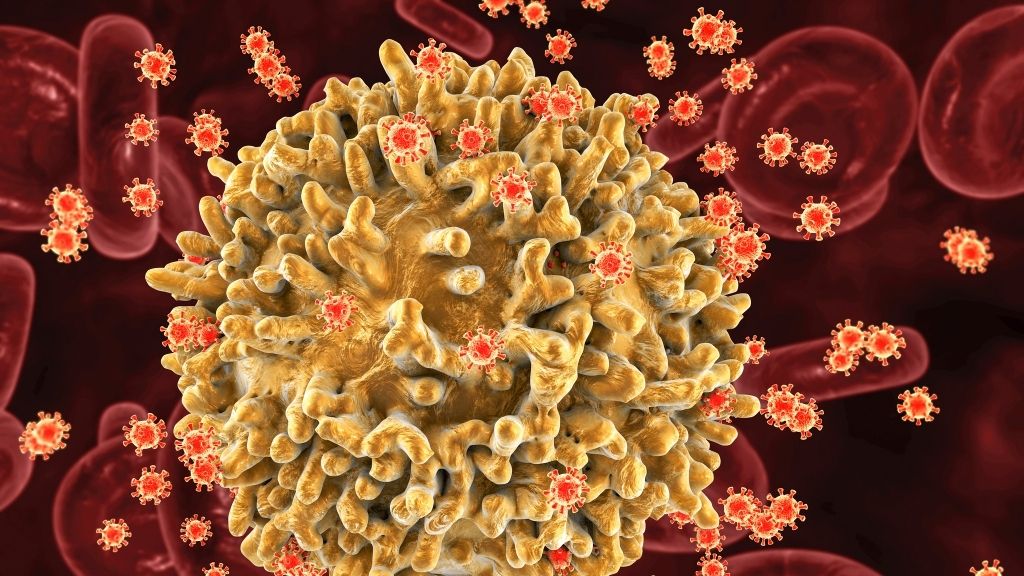 HIV infects immune cells within the frame. (Symbol credit score: KATERYNA KON/SCIENCE PHOTO LIBRARY by the use of Getty Pictures)All about EBT-101According to a up to date presentation at the yearly assembly of the Ecu Society for Gene and Mobile Treatment in Brussels, EBT-101 makes use of more than one guides to focus on more than one websites within the genome and snip out huge sections of latently built-in HIV DNA. This stops HIV from replicating.Kamel Kahlili, a professor of neurovirology and gene enhancing at Temple College and co-founder of Excision BioTherapeutics, has been operating with the corporate to expand EBT-101 for a decade. In 2020 and 2023, Khalili and his group revealed studies that confirmed that EBT-100, a precursor to EBT-101, safely centered and got rid of HIV DNA in inflamed primates.Now, they are checking out their EBT-101 concentrated on technique in people in an early-stage medical trial that essentially makes a speciality of the security of the remedy. Preliminary effects from 3 handled sufferers confirmed no poisonous results or critical opposed occasions. All the sufferers’ HIV is lately suppressed with cART.”The preliminary protection effects are promising as a result of no opposed results had been noticed up to now,” mentioned Hope, whose lab research the mechanisms at the back of HIV infections. “However extra time is wanted to make sure on account of the way in which genetic off-target mutations can take years to manifest into headaches,” he added.Off-target results of CRISPR confer with when the CRISPR molecule alters DNA at websites rather than the ones centered. Those unintentional snips have lengthy been a fear for researchers designing CRISPR therapies, so they are going to be one thing to look forward to with EBT-101, particularly because the remedy goals more than one websites within the genome.Moreover, professionals advised Are living Science that despite the fact that the continued trial hints that the treatment has a favorable protection profile, we nonetheless have no idea whether or not one dose can successfully goal latent HIV cells and whether or not it may keep watch over HIV in people.”It will be important to means this with warning,” Herrera-Carrillo mentioned. “Whilst optimism about being heading in the right direction is justified, it’s a must to acknowledge the really extensive paintings that also must be executed.”The medical trial will subsequent check further doses of EBT-101 for protection after which decide whether or not the virus remains suppressed when sufferers are taken off cART. cART treatments pump the brakes on HIV replication, so the one strategy to decide whether or not the latent cellular reservoirs had been disabled is to quickly carry the ones brakes.Interruptions to HIV remedy are vital to decide whether or not a affected person is in remission — as has took place within the few other folks cured of HIV — however generally, practical remedy interruptions had been debated because of their inherent dangers.Following those exams, EBT-101 trial members shall be enrolled in a long-term follow-up find out about for 15 years following their preliminary dose to test for long-term opposed results. So the knowledge is on its manner, however will take years to reach.Ever marvel why some other folks construct muscle extra simply than others or why freckles pop out within the solar? Ship us your questions on how the human frame works to neighborhood@livescience.com with the topic line “Well being Table Q,” and you might even see your query spoke back at the website online!
HIV infects immune cells within the frame. (Symbol credit score: KATERYNA KON/SCIENCE PHOTO LIBRARY by the use of Getty Pictures)All about EBT-101According to a up to date presentation at the yearly assembly of the Ecu Society for Gene and Mobile Treatment in Brussels, EBT-101 makes use of more than one guides to focus on more than one websites within the genome and snip out huge sections of latently built-in HIV DNA. This stops HIV from replicating.Kamel Kahlili, a professor of neurovirology and gene enhancing at Temple College and co-founder of Excision BioTherapeutics, has been operating with the corporate to expand EBT-101 for a decade. In 2020 and 2023, Khalili and his group revealed studies that confirmed that EBT-100, a precursor to EBT-101, safely centered and got rid of HIV DNA in inflamed primates.Now, they are checking out their EBT-101 concentrated on technique in people in an early-stage medical trial that essentially makes a speciality of the security of the remedy. Preliminary effects from 3 handled sufferers confirmed no poisonous results or critical opposed occasions. All the sufferers’ HIV is lately suppressed with cART.”The preliminary protection effects are promising as a result of no opposed results had been noticed up to now,” mentioned Hope, whose lab research the mechanisms at the back of HIV infections. “However extra time is wanted to make sure on account of the way in which genetic off-target mutations can take years to manifest into headaches,” he added.Off-target results of CRISPR confer with when the CRISPR molecule alters DNA at websites rather than the ones centered. Those unintentional snips have lengthy been a fear for researchers designing CRISPR therapies, so they are going to be one thing to look forward to with EBT-101, particularly because the remedy goals more than one websites within the genome.Moreover, professionals advised Are living Science that despite the fact that the continued trial hints that the treatment has a favorable protection profile, we nonetheless have no idea whether or not one dose can successfully goal latent HIV cells and whether or not it may keep watch over HIV in people.”It will be important to means this with warning,” Herrera-Carrillo mentioned. “Whilst optimism about being heading in the right direction is justified, it’s a must to acknowledge the really extensive paintings that also must be executed.”The medical trial will subsequent check further doses of EBT-101 for protection after which decide whether or not the virus remains suppressed when sufferers are taken off cART. cART treatments pump the brakes on HIV replication, so the one strategy to decide whether or not the latent cellular reservoirs had been disabled is to quickly carry the ones brakes.Interruptions to HIV remedy are vital to decide whether or not a affected person is in remission — as has took place within the few other folks cured of HIV — however generally, practical remedy interruptions had been debated because of their inherent dangers.Following those exams, EBT-101 trial members shall be enrolled in a long-term follow-up find out about for 15 years following their preliminary dose to test for long-term opposed results. So the knowledge is on its manner, however will take years to reach.Ever marvel why some other folks construct muscle extra simply than others or why freckles pop out within the solar? Ship us your questions on how the human frame works to neighborhood@livescience.com with the topic line “Well being Table Q,” and you might even see your query spoke back at the website online!