Abstract: New analysis has exposed a easy neural circuit that connects starvation indicators to jaw actions required for consuming. Scientists recognized a three-neuron pathway involving the hormone leptin and BDNF neurons, which controls the jaw’s motor serve as.When those neurons have been activated, mice stopped consuming; when inhibited, they made chewing motions even with out meals. The findings counsel that feeding habits would possibly resemble a reflex, providing new insights into how starvation and motor management are connected.Key Info:A 3-neuron circuit connects starvation indicators to chewing actions.Inhibiting BDNF neurons reasons compulsive chewing, even with out meals.Feeding habits would possibly perform like a reflex, pushed through this easy neural circuit.Supply: Rockefeller UniversitySpeaking, making a song, coughing, guffawing, yelling, yawning, chewing—we use our jaws for plenty of functions. Each and every motion calls for a fancy coordination of muscle tissue whose task is controlled through neurons within the mind.But it surely seems that the neural circuit in the back of the jaw motion maximum very important to survival—consuming—is strangely easy, as researchers from Rockefeller College lately described in a brand new paper in Nature. Christin Kosse and different scientists from the Laboratory of Molecular Genetics, headed through Jeffrey M. Friedman, have recognized a three-neuron circuit that connects a hunger-signaling hormone to the jaw actions of chewing. 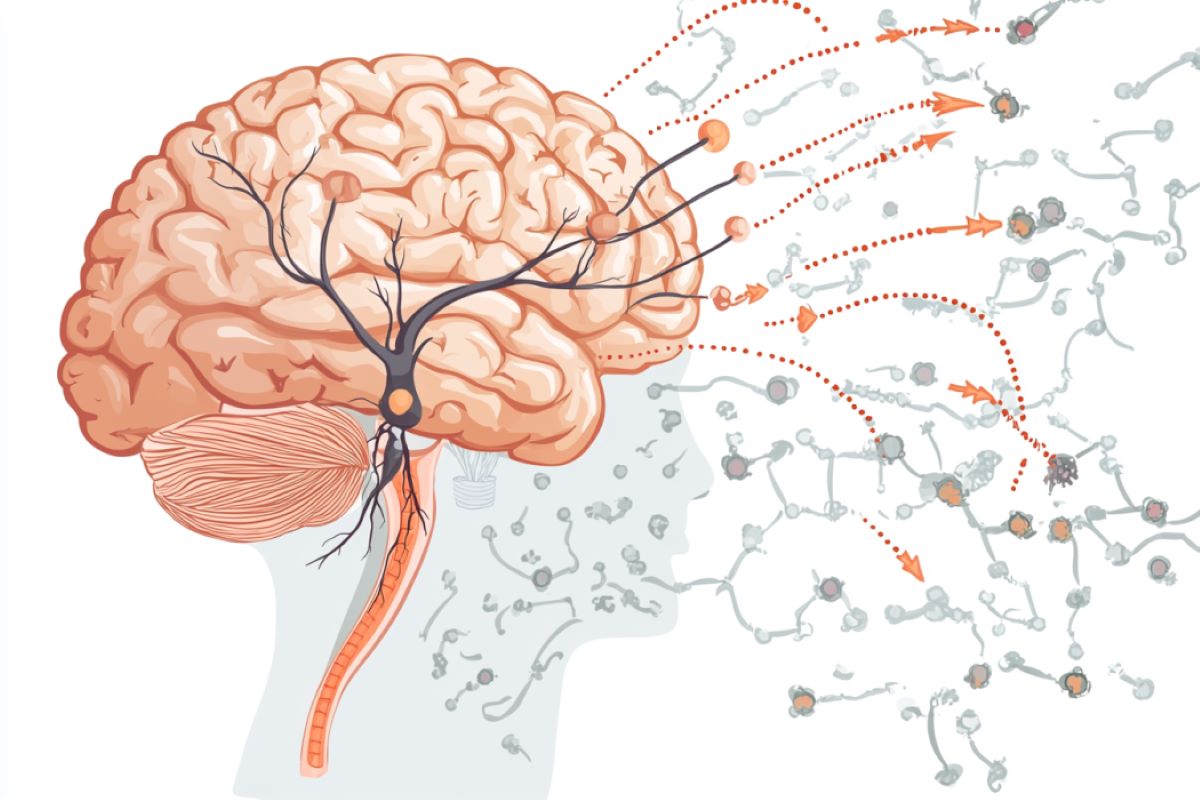 The Arc neurons venture to the ventromedial hypothalamus, the place their indicators are picked up through the BDNF neurons, which then venture at once to a brainstem heart referred to as Me5 that controls the motion of jaw muscle tissue. Credit score: Neuroscience NewsThe middleman between those two is a cluster of neurons in a selected house of the hypothalamus that, when broken, has lengthy been identified to motive weight problems.Strikingly, inhibiting those so-called BDNF neurons no longer handiest leads animals to eat extra meals but in addition triggers the jaw to make chewing motions even within the absence of meals or different sensory enter that might point out it used to be time to consume. And stimulating them reduces meals consumption and places a halt to the chewing motions, performing as an efficient curb towards starvation.The straightforward structure of this circuit means that the impulse to consume is also extra very similar to a reflex than used to be in the past regarded as—and would possibly supply a brand new clue about how the initiation of feeding is managed. “It’s sudden that those neurons are so keyed to motor management,” says find out about first writer Christin Kosse, a analysis affiliate within the lab. “We didn’t be expecting that restricting bodily jaw movement may just act as a type of urge for food suppressant.” Greater than a sense?The impulse to consume is pushed no longer simply by starvation however through many components. We additionally consume for excitement, neighborhood, ritual, and dependancy; and scent, style, and feelings can affect whether or not we consume too. In people, consuming can be regulated through the mindful need to eat kind of.The reasons of weight problems are similarly advanced, the results of a dynamic interaction of nutrition, atmosphere, and genes. For instance, mutations in different genes, together with leptin, the hunger-suppressing hormone, and brain-derived neurotrophic issue (BDNF), result in gross overeating, metabolic adjustments, and excessive weight problems, suggesting that each components in most cases suppress urge for food.When Friedman’s workforce started this find out about, they sought to pinpoint the site of the BDNF neurons that curtail overeating. That’s eluded scientists for years, as a result of BDNF neurons, which might be additionally number one regulators of neuronal building, differentiation, and survival, are common within the mind. Within the present find out about, they homed in at the ventromedial hypothalamus (VMH), a deep-brain area connected to glucose legislation and urge for food. It’s well-documented that injury within the VMH may end up in overeating and sooner or later weight problems in animals and other folks, simply as mutated BDNF proteins do. Most likely the VMH performed a regulatory function in feeding habits.They was hoping that through documenting BDNF’s affect on consuming habits, they might in finding the neural circuit underpinning the method of reworking sensory indicators into jaw motions. They therefore discovered that BDNF neurons within the VMH—however no longer somewhere else—are activated when animals develop into overweight, suggesting that they’re activated when weight is won so as to suppress meals consumption. Thus when those neurons are lacking, or there’s a mutation in BDNF, animals develop into overweight.Chewing with out foodIn a chain of experiments, the researchers then used optogenetics to both specific or inhibit the BDNF neurons within the ventromedial hypothalamus of mice. When the neurons have been activated, the mice totally stopped feeding, even if they have been identified to be hungry.Silencing them had the other impact: the mice started to consume—and consume and consume and consume, gobbling down just about 1200% extra meals than they in most cases would in a brief time frame.“Once we noticed those effects, we to begin with concept that in all probability BDNF neurons encode valence,” Kosse says.“We puzzled if once we regulated those neurons, the mice have been experiencing the detrimental feeling of starvation or perhaps the certain feeling of consuming meals that’s scrumptious.”However next experiments disproved that concept. Without reference to the meals given to the mice—both their same old chow or meals filled with fats and sugar, just like the mouse similar of a chocolate mousse cake—they discovered that activating the BDNF neurons suppressed meals consumption.And since starvation isn’t the one motivation to consume—as any person not able to skip dessert can attest—additionally they presented high-palatable meals to mice that have been already properly fed. The animals chowed down till the researchers inhibited the BDNF neurons, at which level they promptly stopped consuming. “This used to be to begin with a perplexing discovering, as a result of prior research have recommended that this ‘hedonic’ force to consume for excitement is relatively other from the starvation force, which is an try to suppress the detrimental feeling, or detrimental valence, related to starvation through consuming,” Kosse notes.“We demonstrated that activating BDNF neurons can suppress each drives.”Similarly hanging used to be that BDNF inhibition led to the mice to make chewing motions with their jaw, directed at any object of their neighborhood even if meals used to be no longer to be had. This compulsion to bite and chew used to be so robust that the mice gnawed on anything else round them—the steel spout of a water feeder, a block of picket, even the wires tracking their neural task.The circuitBut how does this motor-control transfer connect with the frame’s want or need for meals?Through mapping the inputs and outputs of the BDNF neurons, the researchers came upon that BDNF neurons are the linchpin of a three-part neural circuit linking hormonal indicators that keep watch over urge for food to the actions required to eat it.At one finish of the circuit are particular neurons within the arcuate nucleus (Arc) area of the hypothalamus that pick out up starvation indicators such because the hormone leptin, which is produced through fats cells. (A excessive quantity of leptin method the power tank is complete, whilst a low leptin stage signifies it’s time to consume. Animals without a leptin develop into overweight.)The Arc neurons venture to the ventromedial hypothalamus, the place their indicators are picked up through the BDNF neurons, which then venture at once to a brainstem heart referred to as Me5 that controls the motion of jaw muscle tissue.“Different research have proven that while you kill Me5 neurons in mice throughout building, the animals will starve as a result of they’re not able to bite cast meals,” says Kosse. “So it is sensible that once we manipulate the BDNF neurons projecting there, we see jaw actions.”It additionally explains why injury within the VMH reasons weight problems, Friedman says. “The proof offered in our paper displays that the weight problems related to those lesions is a results of a lack of those BDNF neurons, and the findings unify the identified mutations that motive weight problems right into a somewhat coherent circuit.”The findings counsel one thing deeper concerning the connection between sensation and behaviour, he provides. “The structure of the feeding circuit isn’t very other from the structure of a reflex,” says Friedman.“That’s sudden, as a result of consuming is a fancy habits—one through which many components affect whether or not you’ll start up the habits, however none of them ensure it. However, a reflex is modest: an outlined stimulus and an invariant reaction.“In a way, what this paper displays is that the road between habits and reflex is most definitely extra blurred than we concept. We hypothesize that the neurons on this circuit are the objective of alternative neurons within the mind that put across different indicators that keep watch over urge for food.”This speculation is in step with the paintings of early twentieth century neurophysiologist Charles Sherrington, who identified that whilst cough is regulated through a regular reflex, it may be modulated through mindful components, similar to the will to suppress it in a crowded theater.Kosse provides, “As a result of feeding is so very important to fundamental survival, this circuit regulating meals consumption is also historical. Most likely it used to be a substrate for ever-more advanced processing that took place because the mind advanced.”To that finish, at some point the researchers need to discover the brainstem house referred to as Me5 with the concept that the jaw’s motor controls could be an invaluable fashion for working out different behaviors, together with compulsive, stress-related mouth movements similar to gnawing on a pencil eraser or strands of 1’s hair.“Through analyzing those premotor neurons within the Me5, we could possibly perceive whether or not there are different facilities that venture into the area and affect different innate behaviors, like BDNF neurons do for consuming,” she says. “Are there stress-activated or different neurons that venture into there as properly?”About this neuroscience analysis newsAuthor: Katherine Fenz
The Arc neurons venture to the ventromedial hypothalamus, the place their indicators are picked up through the BDNF neurons, which then venture at once to a brainstem heart referred to as Me5 that controls the motion of jaw muscle tissue. Credit score: Neuroscience NewsThe middleman between those two is a cluster of neurons in a selected house of the hypothalamus that, when broken, has lengthy been identified to motive weight problems.Strikingly, inhibiting those so-called BDNF neurons no longer handiest leads animals to eat extra meals but in addition triggers the jaw to make chewing motions even within the absence of meals or different sensory enter that might point out it used to be time to consume. And stimulating them reduces meals consumption and places a halt to the chewing motions, performing as an efficient curb towards starvation.The straightforward structure of this circuit means that the impulse to consume is also extra very similar to a reflex than used to be in the past regarded as—and would possibly supply a brand new clue about how the initiation of feeding is managed. “It’s sudden that those neurons are so keyed to motor management,” says find out about first writer Christin Kosse, a analysis affiliate within the lab. “We didn’t be expecting that restricting bodily jaw movement may just act as a type of urge for food suppressant.” Greater than a sense?The impulse to consume is pushed no longer simply by starvation however through many components. We additionally consume for excitement, neighborhood, ritual, and dependancy; and scent, style, and feelings can affect whether or not we consume too. In people, consuming can be regulated through the mindful need to eat kind of.The reasons of weight problems are similarly advanced, the results of a dynamic interaction of nutrition, atmosphere, and genes. For instance, mutations in different genes, together with leptin, the hunger-suppressing hormone, and brain-derived neurotrophic issue (BDNF), result in gross overeating, metabolic adjustments, and excessive weight problems, suggesting that each components in most cases suppress urge for food.When Friedman’s workforce started this find out about, they sought to pinpoint the site of the BDNF neurons that curtail overeating. That’s eluded scientists for years, as a result of BDNF neurons, which might be additionally number one regulators of neuronal building, differentiation, and survival, are common within the mind. Within the present find out about, they homed in at the ventromedial hypothalamus (VMH), a deep-brain area connected to glucose legislation and urge for food. It’s well-documented that injury within the VMH may end up in overeating and sooner or later weight problems in animals and other folks, simply as mutated BDNF proteins do. Most likely the VMH performed a regulatory function in feeding habits.They was hoping that through documenting BDNF’s affect on consuming habits, they might in finding the neural circuit underpinning the method of reworking sensory indicators into jaw motions. They therefore discovered that BDNF neurons within the VMH—however no longer somewhere else—are activated when animals develop into overweight, suggesting that they’re activated when weight is won so as to suppress meals consumption. Thus when those neurons are lacking, or there’s a mutation in BDNF, animals develop into overweight.Chewing with out foodIn a chain of experiments, the researchers then used optogenetics to both specific or inhibit the BDNF neurons within the ventromedial hypothalamus of mice. When the neurons have been activated, the mice totally stopped feeding, even if they have been identified to be hungry.Silencing them had the other impact: the mice started to consume—and consume and consume and consume, gobbling down just about 1200% extra meals than they in most cases would in a brief time frame.“Once we noticed those effects, we to begin with concept that in all probability BDNF neurons encode valence,” Kosse says.“We puzzled if once we regulated those neurons, the mice have been experiencing the detrimental feeling of starvation or perhaps the certain feeling of consuming meals that’s scrumptious.”However next experiments disproved that concept. Without reference to the meals given to the mice—both their same old chow or meals filled with fats and sugar, just like the mouse similar of a chocolate mousse cake—they discovered that activating the BDNF neurons suppressed meals consumption.And since starvation isn’t the one motivation to consume—as any person not able to skip dessert can attest—additionally they presented high-palatable meals to mice that have been already properly fed. The animals chowed down till the researchers inhibited the BDNF neurons, at which level they promptly stopped consuming. “This used to be to begin with a perplexing discovering, as a result of prior research have recommended that this ‘hedonic’ force to consume for excitement is relatively other from the starvation force, which is an try to suppress the detrimental feeling, or detrimental valence, related to starvation through consuming,” Kosse notes.“We demonstrated that activating BDNF neurons can suppress each drives.”Similarly hanging used to be that BDNF inhibition led to the mice to make chewing motions with their jaw, directed at any object of their neighborhood even if meals used to be no longer to be had. This compulsion to bite and chew used to be so robust that the mice gnawed on anything else round them—the steel spout of a water feeder, a block of picket, even the wires tracking their neural task.The circuitBut how does this motor-control transfer connect with the frame’s want or need for meals?Through mapping the inputs and outputs of the BDNF neurons, the researchers came upon that BDNF neurons are the linchpin of a three-part neural circuit linking hormonal indicators that keep watch over urge for food to the actions required to eat it.At one finish of the circuit are particular neurons within the arcuate nucleus (Arc) area of the hypothalamus that pick out up starvation indicators such because the hormone leptin, which is produced through fats cells. (A excessive quantity of leptin method the power tank is complete, whilst a low leptin stage signifies it’s time to consume. Animals without a leptin develop into overweight.)The Arc neurons venture to the ventromedial hypothalamus, the place their indicators are picked up through the BDNF neurons, which then venture at once to a brainstem heart referred to as Me5 that controls the motion of jaw muscle tissue.“Different research have proven that while you kill Me5 neurons in mice throughout building, the animals will starve as a result of they’re not able to bite cast meals,” says Kosse. “So it is sensible that once we manipulate the BDNF neurons projecting there, we see jaw actions.”It additionally explains why injury within the VMH reasons weight problems, Friedman says. “The proof offered in our paper displays that the weight problems related to those lesions is a results of a lack of those BDNF neurons, and the findings unify the identified mutations that motive weight problems right into a somewhat coherent circuit.”The findings counsel one thing deeper concerning the connection between sensation and behaviour, he provides. “The structure of the feeding circuit isn’t very other from the structure of a reflex,” says Friedman.“That’s sudden, as a result of consuming is a fancy habits—one through which many components affect whether or not you’ll start up the habits, however none of them ensure it. However, a reflex is modest: an outlined stimulus and an invariant reaction.“In a way, what this paper displays is that the road between habits and reflex is most definitely extra blurred than we concept. We hypothesize that the neurons on this circuit are the objective of alternative neurons within the mind that put across different indicators that keep watch over urge for food.”This speculation is in step with the paintings of early twentieth century neurophysiologist Charles Sherrington, who identified that whilst cough is regulated through a regular reflex, it may be modulated through mindful components, similar to the will to suppress it in a crowded theater.Kosse provides, “As a result of feeding is so very important to fundamental survival, this circuit regulating meals consumption is also historical. Most likely it used to be a substrate for ever-more advanced processing that took place because the mind advanced.”To that finish, at some point the researchers need to discover the brainstem house referred to as Me5 with the concept that the jaw’s motor controls could be an invaluable fashion for working out different behaviors, together with compulsive, stress-related mouth movements similar to gnawing on a pencil eraser or strands of 1’s hair.“Through analyzing those premotor neurons within the Me5, we could possibly perceive whether or not there are different facilities that venture into the area and affect different innate behaviors, like BDNF neurons do for consuming,” she says. “Are there stress-activated or different neurons that venture into there as properly?”About this neuroscience analysis newsAuthor: Katherine Fenz
Supply: Rockefeller College
Touch: Katherine Fenz – Rockefeller College
Symbol: The picture is credited to Neuroscience NewsOriginal Analysis: Open get right of entry to.
“A subcortical feeding circuit linking an interoceptive node to jaw motion” through Christin Kosse et al. NatureAbstractA subcortical feeding circuit linking an interoceptive node to jaw movementThe mind processes an array of stimuli, enabling the number of suitable behavioural responses, however the neural pathways linking interoceptive inputs to outputs for feeding are poorly understood.Right here we delineate a subcortical circuit through which brain-derived neurotrophic issue (BDNF)-expressing neurons within the ventromedial hypothalamus (VMH) at once attach interoceptive inputs to motor centres, controlling meals intake and jaw actions.VMHBDNF neuron inhibition will increase meals consumption through gating motor sequences of feeding via projections to premotor spaces of the jaw. When meals is unavailable, VMHBDNF inhibition elicits consummatory behaviours directed at inanimate items similar to wood blocks, and inhibition of perimesencephalic trigeminal house (pMe5) projections inspires rhythmic jaw actions.The task of those neurons is lowered throughout meals intake and will increase when meals is in proximity however no longer ate up. Job may be larger in overweight animals and after leptin remedy. VMHBDNF neurons obtain monosynaptic inputs from each agouti-related peptide (AgRP) and proopiomelanocortin neurons within the arcuate nucleus (Arc), and constitutive VMHBDNF activation blocks the orexigenic impact of AgRP activation.Those knowledge point out an Arc → VMHBDNF → pMe5 circuit that senses the power state of an animal and regulates consummatory behaviours in a state-dependent approach.
Neural Circuit Discovered to Keep an eye on Chewing and Urge for food – Neuroscience Information




:max_bytes(150000):strip_icc()/GettyImages-2188460679-4f112c9e9def4df98120dc4919bbd105.jpg)






