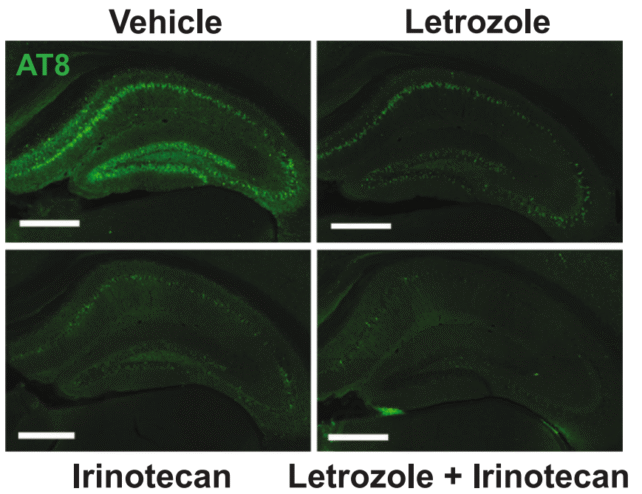
Eisai-Biogen drug first remedy to gradual Alzheimer’s courseUK draft steering on price a blow after gradual take-up in U.S.Aug 22 (Reuters) – Alzheimer’s sufferers in Britain’s state-run well being provider are not going to get get right of entry to to Eisai (4523.T), opens new tab and Biogen’s (BIIB.O), opens new tab new Leqembi drug, after it used to be authorized on Thursday via the rustic’s regulator however deemed too pricey for broad use.The Medications and Healthcare merchandise Regulatory Company (MHRA) mentioned the drug, sometimes called lecanemab, is the primary remedy for Alzheimer’s authorized to be used within the nation that displays some proof of slowing the development of the illness.However in draft steering printed concurrently, the Nationwide Institute for Well being and Care Excellence (NICE), mentioned the medication’s top price and wish for in depth tracking for unwanted effects “approach it can’t be regarded as excellent worth for the taxpayer”.NICE additionally cited a loss of proof at the long-term results of taking the remedy, noting that the medical trial handiest reported results when other folks were taking lecanemab for 18 months.The United Kingdom price effectiveness frame’s findings mark the newest blow confronted via the drugmakers amid gradual take-up of the drug within the U.S. because of the price and issues over unwanted effects and efficacy.It additionally highlights the complexities of a brand new elegance of substances that receive advantages early-stage Alzheimer’s sufferers however elevate the chance of uncommon and severe unwanted effects.NICE’s steering at the drug is open for public session till Sept. 20, and a last advice will apply its analysis of the responses.NICE additionally mentioned that round 70,000 adults in England would were eligible for remedy with drug.Eisai and Biogen mentioned they’re running with NICE, the Scottish Medications Consortium and the Nationwide Well being Carrier to make Leqembi to be had “once imaginable.”Stocks of Biogen had been down 1.3% at $203.52 in early buying and selling.Professor Paul Morgan, Intervening time Director of the United Kingdom Dementia Analysis Institute Cardiff, at Cardiff College in Wales, mentioned that whilst NICE’s determination would disappoint other folks with top hopes for the brand new treatment, the frame’s “wait and notice” manner used to be comprehensible given what he known as “unanswered questions” in regards to the long-term have an effect on and unwanted effects.Every other dementia professional, Professor Vanessa Raymont, an affiliate professor within the psychiatry division on the College of Oxford, mentioned that whilst new therapies for Alzheimer’s are essential, blood checks to diagnose the memory-robbing illness are similarly as essential.Mavens and corporate executives instructed Reuters closing 12 months that whilst a number of such checks are in construction, it’ll be any other couple of years prior to they turn into an on a regular basis device.SIDE EFFECTSLecanemab slowed development of Alzheimer’s illness via 27% when put next with a placebo, in a big trial whose results had been reported via the firms in 2022.The treatment has been authorized in the US, China, Hong Kong, Israel, Japan, South Korea and the UAE.However closing month the Eu Union’s medicine regulator rejected the drug, announcing the chance of great mind swelling didn’t outweigh its small have an effect on on slowing cognitive decline.The firms mentioned then that they’d search re-assessment of the advice, however didn’t expose what data they’d give you the regulator.An approval in Britain is a vital step in understanding the estimated $1.6 billion in income from Europe for Leqembi, “a essential approval this is had to additional boost up gross sales for the drug,” BMO Capital Markets analyst Evan Seigerman mentioned.The infusion, given two times a month, gets rid of sticky clumps of protein amyloid beta from the mind, believed to be a trademark of Alzheimer’s illness.It’s been related to severe unwanted effects for some sufferers, together with mind swelling and bleeding or microhemorrhages.Leqembi remedy is priced at $26,500 a 12 months within the U.S. In Britain, the NICE draft steering said that the fee is confidential till printed via the United Kingdom’s Division for Well being and Social Care.For now, excluding Leqembi, there is just one different Alzheimer’s drug available on the market designed to gradual the process the illness: Eli Lilly’s (LLY.N), opens new tab drug donanemab, authorized via the U.S. Meals and Drug Management closing month. Join right here.Reporting via Maggie Fick in London and Prerna Bedi in Bengaluru, further reporting via Sneha S Ok; Enhancing via Sherry Jacob-Phillips and Jane MerrimanOur Requirements: The Thomson Reuters Agree with Ideas., opens new tabPurchase Licensing Rights Maggie is a Britain-based reporter overlaying the Eu prescribed drugs trade with a world viewpoint. In 2023, Maggie’s protection of Danish drugmaker Novo Nordisk and its race to extend manufacturing of its new weight-loss drug helped the Well being & Pharma group win a Reuters Newshounds of the 12 months award within the Beat Protection of the 12 months class. Since November 2023, she has additionally been collaborating in Reuters protection associated with the Israel-Hamas battle. Up to now founded in Nairobi and Cairo for Reuters and in Lagos for the Monetary Instances, Maggie were given her get started in journalism in 2010 as a freelancer for The Related Press in South Sudan.
Maggie is a Britain-based reporter overlaying the Eu prescribed drugs trade with a world viewpoint. In 2023, Maggie’s protection of Danish drugmaker Novo Nordisk and its race to extend manufacturing of its new weight-loss drug helped the Well being & Pharma group win a Reuters Newshounds of the 12 months award within the Beat Protection of the 12 months class. Since November 2023, she has additionally been collaborating in Reuters protection associated with the Israel-Hamas battle. Up to now founded in Nairobi and Cairo for Reuters and in Lagos for the Monetary Instances, Maggie were given her get started in journalism in 2010 as a freelancer for The Related Press in South Sudan.
New Alzheimer’s drug deemed too pricey for UK’s state-run well being provider