Abstract: Researchers have exposed a mechanism that can cause ALS’s earliest levels, figuring out proteins that mislocalize, inflicting neuron degeneration. Via focused on the RNA-binding protein SmD1, scientists have been ready to forestall key ALS proteins from leaving their protecting cell zones, maintaining neuron serve as.The findings would possibly result in ALS remedies able to halting development earlier than important neurodegeneration happens, providing doable new methods in opposition to the illness.Key Details:ALS development would possibly start when the protein CHMP7 mislocalizes, beginning neuron injury.Restoring SmD1 serve as may save you CHMP7 mislocalization and give protection to neurons.The staff hopes that remedies like risdiplam would possibly gradual ALS onset through stabilizing neuron integrity.Supply: UCSDApproximately 5,000 other people within the U.S. broaden amyotrophic lateral sclerosis (ALS) each and every 12 months. On reasonable, they live to tell the tale for best two to 5 years after being recognized, consistent with the Facilities for Illness Keep watch over and Prevention.The hastily progressing neurodegenerative illness reasons the demise of neurons within the mind and spinal twine, leading to muscle weak spot, breathing failure and dementia.In spite of the devastating nature of the illness, little is understood about what first triggers the deterioration of motor neurons on the onset of ALS. 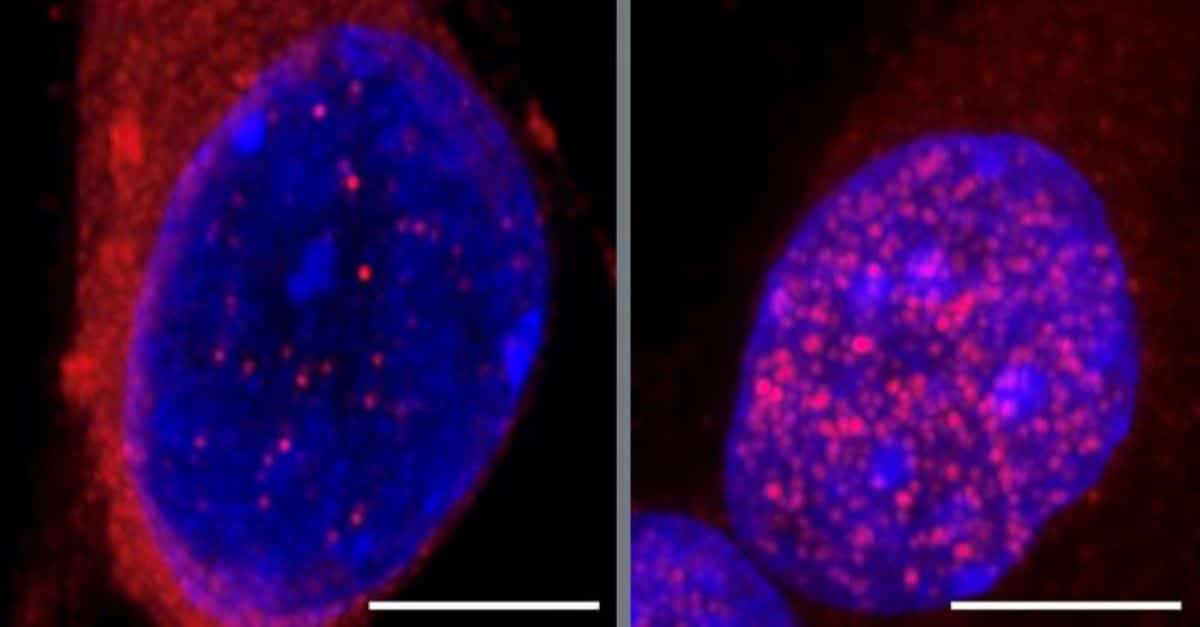 Pictured are nuclei of triggered pluripotent stem cellular (iPSC)-derived motor neurons stained for distinction. The nucleus at the left has been handled with DMSO (keep an eye on) and looks most commonly blue. The nucleus at the proper has been handled with an SMN inhibitor and is noticed purple, indicating that CHMP7 protein has accrued within the nucleus. Scale bars =10 µm. Credit score: UCSDNow, researchers from College of California San Diego and their colleagues file that they have got recognized a key pathway that units off neurodegeneration within the early levels of the illness.The findings may result in construction of remedies to forestall or gradual the development of ALS early on, earlier than primary injury has been accomplished.The learn about was once printed on October 31, 2024 in Neuron.A protein known as TDP-43 is normally situated within the nucleus of motor neurons, the place it regulates gene expression required for the cells to serve as. Research have proven that once TDP-43 as an alternative accumulates within the cytoplasm, out of doors of the nucleus, this is a telltale signal of ALS. How the protein results in the fallacious position, resulting in neuronal degeneration, has stumped researchers till now.“By the point you notice a affected person with ALS and you notice the TDP-43 protein aggregated within the cytoplasm, it’s just like the twist of fate web page with all of the vehicles crashed already, however that’s no longer the beginning tournament,” mentioned corresponding creator Gene Yeo, Ph.D., professor within the Division of Mobile and Molecular Medication at UC San Diego College of Medication and director of the Heart for RNA Applied sciences and Therapeutics and the Sanford Stem Cellular Institute Innovation Heart.Tracing the occasions main as much as the “twist of fate”, Yeo explains that every other protein, known as CHMP7 — most often discovered within the cytoplasm — builds up within the nucleus as an alternative, environment off a cascade of occasions that in the end result in motor neuron degeneration. However what reasons CHMP7 to acquire within the nucleus to start with?Yeo and his staff screened for RNA-binding proteins that would possibly affect CHMP7 build-up within the nucleus. This yielded 55 proteins, 23 of which had a possible connection to ALS pathogenesis. Inhibiting the manufacturing of a number of of those proteins resulted in an build up in CHMP7 within the nucleus.Additional experiments with motor neurons made from ALS-patient-derived triggered pluripotent stem cells resulted within the sudden discovery that depleting such a, an RNA-splicing related protein known as SmD1, which had no longer prior to now been identified to have an effect on CHMP7 ranges, resulted in the most important uptick in its nuclear accumulation.A build-up of CHMP7 within the nucleus damages nucleoporins, which Yeo likens to tiny portals within the membrane setting apart the nucleus from the cytoplasm that orchestrate the motion of proteins and RNA between the 2 cell areas. Dysfunctional nucleoporins permit TDP-43 to go out the nucleus and gather within the cytoplasm. As soon as there, the protein can not oversee the gene expression techniques vital for neurons to serve as.Then again, when the researchers boosted SmD1 expression in cells, CHMP7 was once restored to its standard location within the cytoplasm, leaving nucleopores intact, permitting TDP-43 to stick within the nucleus, thus sparing the motor neurons from degeneration.“You’ll be able to in reality repair the localization of this CHMP7 protein and due to this fact the entire downstream results,” mentioned Norah Al-Azzam, first creator of the learn about, then a neurosciences scholar within the Yeo lab who went directly to earn her Ph.D. in spring 2024.What’s extra, the SmD1 protein is a part of SMN, a multiprotein advanced. SMN disorder is implicated in every other neurodegenerative dysfunction, spinal muscular atrophy.“We’re intrigued as a result of there are in reality therapeutics for spinal muscular atrophy,” Yeo mentioned. “Considered one of them, risdiplam, is a small molecule compound that complements the splicing and expression of SMN2, a gene carefully associated with the SMN1 gene that turns into dysfunctional in ALS.”This hints on the chance that the use of risdiplam to boost SMN ranges may save you ALS from growing previous the earliest degree of the illness.“It’s no longer like all of the neurons die directly,” mentioned Yeo. “Some neurons die first, after which there may be unfold throughout different neurons. Possibly once you get signs, shall we deal with the affected person in order that the remainder of the neurons don’t crash and hope you forestall the development of ALS.”The researchers assume the SMN advanced may play a a very powerful position within the onset of ALS, however additional analysis is wanted. The following steps might be to boost finances to proceed the analysis in animal fashions and in different genetic fashions of ALS, and sooner or later check the effectiveness of risdiplam or different compounds for short-circuiting ALS.Further co-authors at the learn about come with: Jenny H. To, Vaishali Gautam, Dylan C. Lam, Chloe B. Nguyen, Jack T. Naritomi, Assael A. Madrigal, Benjamin Lee, Anthony Avina, Orel Mizrahi, Jasmine R. Mueller, Willard Ford, Anthony Q. Vu, Steven M. Blue, Yashwin L. Madakamutil, Uri Manor, Cara R. Schiavon and Elena Rebollo, all at UC San Diego; Wenhao Jin at Sanford Laboratories for Leading edge Drugs; Lena A. Side road and Marko Jovanovic at Columbia College; Jeffrey D. Rothstein and Alyssa N. Coyne at Johns Hopkins College College of Medication.Investment: The learn about was once funded, partially, through the Nationwide Institutes of Well being (grants R01HG004659, U24 HG009889, R35GM128802, R01AG071869 and R01HG012216), the Nationwide Science Basis (MCB-2224211), and the Chan-Zuckerberg Initiative.About this ALS analysis newsAuthor: Susanne Bard
Pictured are nuclei of triggered pluripotent stem cellular (iPSC)-derived motor neurons stained for distinction. The nucleus at the left has been handled with DMSO (keep an eye on) and looks most commonly blue. The nucleus at the proper has been handled with an SMN inhibitor and is noticed purple, indicating that CHMP7 protein has accrued within the nucleus. Scale bars =10 µm. Credit score: UCSDNow, researchers from College of California San Diego and their colleagues file that they have got recognized a key pathway that units off neurodegeneration within the early levels of the illness.The findings may result in construction of remedies to forestall or gradual the development of ALS early on, earlier than primary injury has been accomplished.The learn about was once printed on October 31, 2024 in Neuron.A protein known as TDP-43 is normally situated within the nucleus of motor neurons, the place it regulates gene expression required for the cells to serve as. Research have proven that once TDP-43 as an alternative accumulates within the cytoplasm, out of doors of the nucleus, this is a telltale signal of ALS. How the protein results in the fallacious position, resulting in neuronal degeneration, has stumped researchers till now.“By the point you notice a affected person with ALS and you notice the TDP-43 protein aggregated within the cytoplasm, it’s just like the twist of fate web page with all of the vehicles crashed already, however that’s no longer the beginning tournament,” mentioned corresponding creator Gene Yeo, Ph.D., professor within the Division of Mobile and Molecular Medication at UC San Diego College of Medication and director of the Heart for RNA Applied sciences and Therapeutics and the Sanford Stem Cellular Institute Innovation Heart.Tracing the occasions main as much as the “twist of fate”, Yeo explains that every other protein, known as CHMP7 — most often discovered within the cytoplasm — builds up within the nucleus as an alternative, environment off a cascade of occasions that in the end result in motor neuron degeneration. However what reasons CHMP7 to acquire within the nucleus to start with?Yeo and his staff screened for RNA-binding proteins that would possibly affect CHMP7 build-up within the nucleus. This yielded 55 proteins, 23 of which had a possible connection to ALS pathogenesis. Inhibiting the manufacturing of a number of of those proteins resulted in an build up in CHMP7 within the nucleus.Additional experiments with motor neurons made from ALS-patient-derived triggered pluripotent stem cells resulted within the sudden discovery that depleting such a, an RNA-splicing related protein known as SmD1, which had no longer prior to now been identified to have an effect on CHMP7 ranges, resulted in the most important uptick in its nuclear accumulation.A build-up of CHMP7 within the nucleus damages nucleoporins, which Yeo likens to tiny portals within the membrane setting apart the nucleus from the cytoplasm that orchestrate the motion of proteins and RNA between the 2 cell areas. Dysfunctional nucleoporins permit TDP-43 to go out the nucleus and gather within the cytoplasm. As soon as there, the protein can not oversee the gene expression techniques vital for neurons to serve as.Then again, when the researchers boosted SmD1 expression in cells, CHMP7 was once restored to its standard location within the cytoplasm, leaving nucleopores intact, permitting TDP-43 to stick within the nucleus, thus sparing the motor neurons from degeneration.“You’ll be able to in reality repair the localization of this CHMP7 protein and due to this fact the entire downstream results,” mentioned Norah Al-Azzam, first creator of the learn about, then a neurosciences scholar within the Yeo lab who went directly to earn her Ph.D. in spring 2024.What’s extra, the SmD1 protein is a part of SMN, a multiprotein advanced. SMN disorder is implicated in every other neurodegenerative dysfunction, spinal muscular atrophy.“We’re intrigued as a result of there are in reality therapeutics for spinal muscular atrophy,” Yeo mentioned. “Considered one of them, risdiplam, is a small molecule compound that complements the splicing and expression of SMN2, a gene carefully associated with the SMN1 gene that turns into dysfunctional in ALS.”This hints on the chance that the use of risdiplam to boost SMN ranges may save you ALS from growing previous the earliest degree of the illness.“It’s no longer like all of the neurons die directly,” mentioned Yeo. “Some neurons die first, after which there may be unfold throughout different neurons. Possibly once you get signs, shall we deal with the affected person in order that the remainder of the neurons don’t crash and hope you forestall the development of ALS.”The researchers assume the SMN advanced may play a a very powerful position within the onset of ALS, however additional analysis is wanted. The following steps might be to boost finances to proceed the analysis in animal fashions and in different genetic fashions of ALS, and sooner or later check the effectiveness of risdiplam or different compounds for short-circuiting ALS.Further co-authors at the learn about come with: Jenny H. To, Vaishali Gautam, Dylan C. Lam, Chloe B. Nguyen, Jack T. Naritomi, Assael A. Madrigal, Benjamin Lee, Anthony Avina, Orel Mizrahi, Jasmine R. Mueller, Willard Ford, Anthony Q. Vu, Steven M. Blue, Yashwin L. Madakamutil, Uri Manor, Cara R. Schiavon and Elena Rebollo, all at UC San Diego; Wenhao Jin at Sanford Laboratories for Leading edge Drugs; Lena A. Side road and Marko Jovanovic at Columbia College; Jeffrey D. Rothstein and Alyssa N. Coyne at Johns Hopkins College College of Medication.Investment: The learn about was once funded, partially, through the Nationwide Institutes of Well being (grants R01HG004659, U24 HG009889, R35GM128802, R01AG071869 and R01HG012216), the Nationwide Science Basis (MCB-2224211), and the Chan-Zuckerberg Initiative.About this ALS analysis newsAuthor: Susanne Bard
Supply: UCSD
Touch: Susanne Bard – UCSD
Symbol: The picture is credited to UCSDOriginal Analysis: Open get right of entry to.
“Inhibition of RNA splicing triggers CHMP7 nuclear access, impacting TDP-43 serve as and resulting in the onset of ALS cell phenotypes” through Gene Yeo et al. NeuronAbstractInhibition of RNA splicing triggers CHMP7 nuclear access, impacting TDP-43 serve as and resulting in the onset of ALS cell phenotypesAmyotrophic lateral sclerosis (ALS) is connected to the aid of sure nucleoporins in neurons. Larger nuclear localization of charged multivesicular frame protein 7 (CHMP7), a protein fascinated about nuclear pore surveillance, has been recognized as a key issue harmful nuclear pores and disrupting shipping.The use of CRISPR-based microRaft, adopted through gRNA id (CRaft-ID), we found out 55 RNA-binding proteins (RBPs) that affect CHMP7 localization, together with SmD1, a survival of motor neuron (SMN) advanced element.Immunoprecipitation-mass spectrometry (IP-MS) and enhanced crosslinking and immunoprecipitation (CLIP) analyses printed CHMP7’s interactions with SmD1, small nuclear RNAs, and splicing issue mRNAs in motor neurons (MNs).ALS triggered pluripotent stem cellular (iPSC)-MNs display lowered SmD1 expression, and inhibiting SmD1/SMN advanced larger CHMP7 nuclear localization.Crucially, overexpressing SmD1 in ALS iPSC-MNs restored CHMP7’s cytoplasmic localization and corrected STMN2 splicing. Our findings recommend that early ALS pathogenesis is pushed through SMN advanced dysregulation.
Pathway Recognized for Early Intervention in ALS Development – Neuroscience Information