About 90 years in the past, American chemical engineer Linus Pauling proposed a singular thought. He prompt that two atoms don’t essentially require two or extra electrons to shape a bond, one electron covalent bond too can exist.
Again then Pauling didn’t have get entry to to the equipment required to watch such bonds. His principle used to be confirmed proper in 1998 when scientists found out a unmarried electron bond in phosphorus for the primary time.
Since then such bonds have additionally been detected in hydrogen and different components, however no scientists ever got here throughout a unmarried electron carbon-carbon bond.
Alternatively, for the primary time, a group of researchers from Japan’s Hokkaido College has known a unmarried electron covalent bond between two carbon atoms. Some professionals see this discovery as miracle.
Separating a unmarried electron carbon bond
Two carbon atoms have a tendency to shape bonds with two electrons sharing. Unmarried electron covalent bonds are extremely reactive and no more strong as they have got low bond power. Some of these components make it very difficult to isolate carbon atoms bonded by way of a unmarried electron.
For this reason, “Despite the fact that a number of pioneering research have reported one-electron bonds between heteroatoms, direct proof for one-electron bonds between carbon atoms stays elusive,” the find out about authors notice.
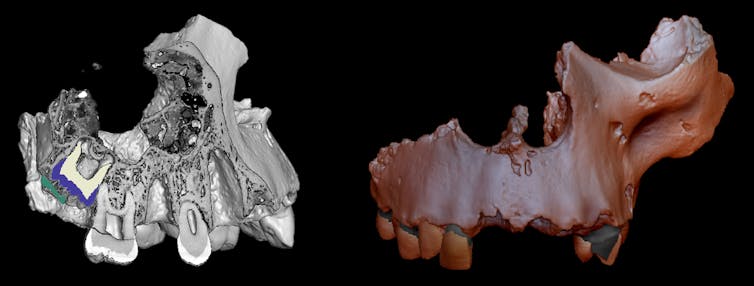
The one imaginable means to do that is to create a compound that might stabilize the bond. To reach this, the find out about authors carried out an oxidation response of a spinoff of hexaphenylethane the usage of iodine. This spinoff contained an excessively elongated paired-electron covalent bond between two carbon atoms.
The response resulted within the formation of violet-colored crystals. When the researchers used X-ray diffraction to inspect the crystals, they spotted that the 2 elongated carbon atoms had come nearer because of the formation of a single-electron covalent between them.
Those effects have been additional checked the usage of Raman spectroscopy, one way used to check the molecular make-up and association of fabrics. The result of this research showed the presence of the one electron C-C bond.
“Those effects thus represent the primary piece of experimental proof for a carbon-carbon single-electron covalent bond, which can also be anticipated to pave the best way for additional traits of the chemistry of this scarcely-explored form of bonding,” Takuya Shimajiri, first creator of the find out about and an assistant professor at Tokyo College, stated.
The importance of this discovery
Carbon is the very important factor for existence and many of the issues that strengthen existence on Earth. From the cells for your frame to the auto you pressure, and the meals you consume, just about the whole thing you spot and use is manufactured from carbon.
The present discovering holds significance as “elucidating the character of single-electron sigma-bonds between two carbon atoms is very important to achieve a deeper working out of chemical-bonding theories and would offer additional insights into chemical reactions,” Yusuke Ishigaki, one of the most find out about authors and a professor at Hokkaido College, stated.
Alternatively, the find out about authors aren’t positive whether or not their analysis may lead to some sensible programs, however they do counsel that “it is going to be within the textbooks.”
The find out about is printed within the magazine Nature.
Science sees miracle as first-ever unmarried electron carbon bond noticed