Using a clever mix of light-triggered chemical reactions, a team of researchers has managed to insert a thread-like molecule into a ring-shaped molecule in a high-energy arrangement — this “molecular fit” is something that can’t happen naturally.
What makes this experiment special is that it created a structure that is not in thermodynamic equilibrium, which is a rare achievement when it comes to artificial systems.
An artificial system like a nanomotor is formed when its molecular components self-assemble and reach a state of thermodynamic equilibrium. However, when it comes to living beings, they function while staying away from self-assembly and thermodynamic equilibrium.
Preventing self-assembly and “reproducing such (living being-like) mechanisms with artificial systems is a complex and ambitious challenge that, if met, could enable the creation of new substances, which could be used to develop, for example, smart drugs and active materials,” the researchers said.
Disrupting the self-assembly of an artificial system
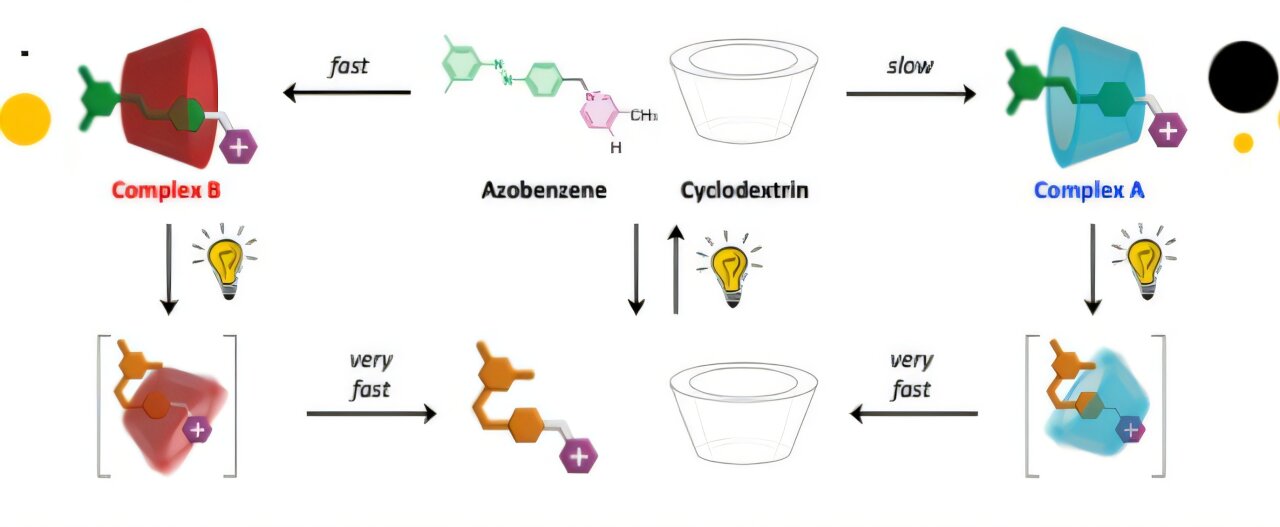 A diagram showing the formation of complex A and complex B. Source: University of Bologna
A diagram showing the formation of complex A and complex B. Source: University of Bologna
The study authors used two molecules for their experiment; the ring-shaped cyclodextrin and a filiform (thread-like) azobenzene derivative. The former is made of glucose, it is used as a drug carrier, food additive, and purification agent. Whereas the latter has the uncanny ability to change shape when exposed to light.
When both molecules interacted in water they resulted in the formation of two complexes; complex A and complex B. Out of the two, A is thermodynamically stable, forms slowly, and can exist even in the absence of light.
However, when the two molecules interacted in the presence of visible light, the azobenzene molecule bends, causing the complex to break apart because its new shape no longer fits the cyclodextrin cavity.
This disruption in the self-assembly of the system resulted in the formation of fast-forming but less stable complex B. The short-lasting unstable complex B represents a novel product that can’t be created when an artificial system follows its original nature.
“The self-assembly mechanism coupled with a photochemical reaction makes it possible to harness the energy of light to accumulate unstable products,” the study authors said.
A secret to developing novel devices
When the light is switched off, azobenzene returns to its original shape, and complex A is formed. You won’t see any sign of the thermodynamically unfavored complex B.
However, this experiment has demonstrated that it is possible to create novel artificial systems by manipulating their self-assembly mechanism.
For instance, the researchers claim that by using their approach and improving it further, one can develop new chemical synthesis methods and devices like nanomotors that can work in non-equilibrium conditions.
“The simplicity and versatility of our approach, together with the fact that visible light – i.e., sunlight – is a clean and sustainable energy source, allow us to foresee developments in various areas of technology and medicine,” Alberto Credi, one of the study authors and a professor at the University of Bologna, said.
The study is published in the journal Chem.