Contemporary advances in molecular biology have supplied remarkable insights into the construction of amyloid fibrils related to Huntington’s illness (HD). Via integrating experimental strategies comparable to cryogenic electron microscopy (cryo-EM) and solid-state nuclear magnetic resonance (ssNMR), researchers are unraveling the advanced structure of protein aggregates that give a contribution to neurodegenerative problems. Those findings are crucial for creating new diagnostic equipment and doable therapies for this incurable illness.Huntington’s illness arises from a genetic mutation that ends up in an odd enlargement of the CAG trinucleotide series within the huntingtin (HTT) gene. This mutation reasons the huntingtin protein to increase a polyglutamine (polyQ) tract that aggregates into amyloid fibrils. Not like the fibrils related to Alzheimer’s or Parkinson’s sicknesses, the ones shaped through polyQ proteins provide distinctive structural demanding situations.PolyQ segments lack strong secondary buildings of their local state, present as intrinsically disordered areas. On the other hand, all the way through illness development, proteolytic cleavage and aberrant splicing of HTT generate N-terminal fragments containing polyQ expansions. Those fragments mixture into amyloid fibrils, forming cell inclusions which are hallmarks of HD pathology. The propensity of polyQ proteins to mixture correlates with tract period, which inversely pertains to the age of illness onset.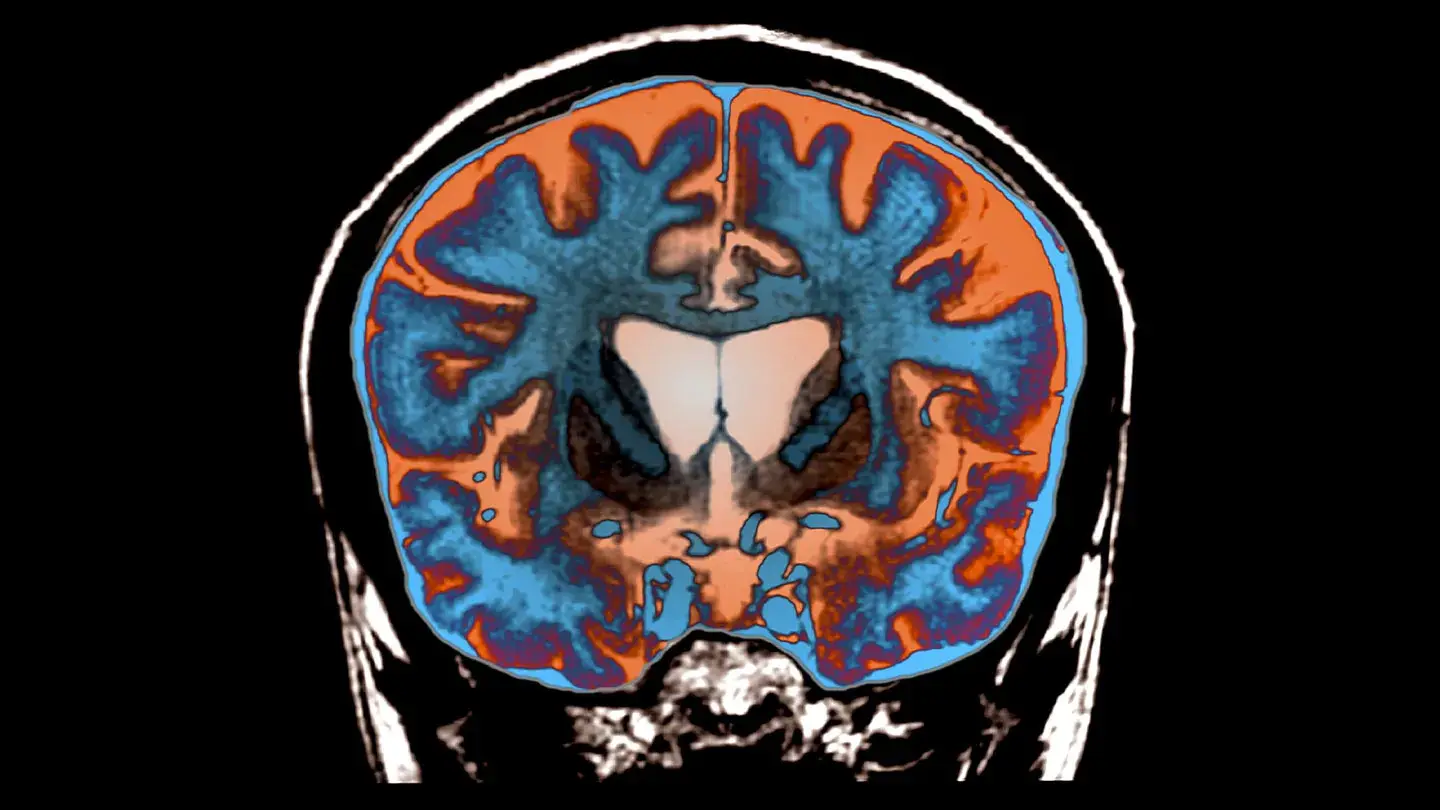 New findings disclose the atomic construction of Huntington’s illness protein aggregates, providing insights into their position in neurodegeneration and doable treatments. (CREDIT: Zephyr/Science Supply) In spite of intensive analysis, the atomic-level construction of polyQ fibrils has remained elusive, essentially because of their repetitive sequences and distinct aggregation patterns. Those fibrils fluctuate from different amyloids through that includes antiparallel β-sheets and block-like cores, making them in particular difficult to check the use of conventional ways. Researchers have lengthy sought to conquer those obstacles to release insights crucial to working out and treating HD.To handle those demanding situations, a staff of world researchers led through Markus Miettinen on the College of Bergen hired integrative structural biology. Via combining information from ssNMR, cryo-EM, and complex computational modeling, they reconstructed the atomic-resolution construction of polyQ and HTTex1 fibrils. This interdisciplinary method bridges experimental and theoretical strategies, providing a complete working out of those distinctive protein clumps.The analysis published that the polyQ fibril core is composed of stacked β-sheets, each and every shaped through antiparallel β-strands with minimum turns. Those sheets are tightly packed, devoid of water, and showcase a repetitive, pseudo-symmetric association. Not like different amyloid fibrils, polyQ buildings lack the twisting patterns frequently noticed in parallel in-register β-sheets. As a substitute, their structure is characterised through “glutamine zippers” and hydrogen-bonded ladders, which stabilize the fibril core.The usage of molecular dynamics simulations and ssNMR information, researchers recognized two dominant glutamine conformations inside the fibril core. Those conformations, known as “a” and “b,” change in a repeating trend, growing a particular spectroscopic fingerprint. This fingerprint lets in for exact characterization of the fibril construction and gives doable goals for diagnostic imaging.The molecular modeling published that the polyQ core is shaped through an infinitely repeating eight-glutamine unit. This structural motif is notable for its translational symmetry and the absence of the twisting generally noticed in amyloid fibrils. Moreover, the construction fulfills crucial constraints required for steadiness, together with particular dihedral angles that allow interdigitating hydrogen bonds between aspect chains. Those findings underscore the individuality of HD-related fibrils and their deviation from typical amyloid buildings.Figuring out the construction of HTTex1 fibrils has vital implications for HD analysis. The molecular structure supplies insights into the organic houses of those fibrils, together with their resistance to degradation and their position in neurotoxicity. For example, the densely packed core and restricted solvent accessibility obstruct post-aggregation polyubiquitination, complicating the cell mechanisms for fibril clearance.
New findings disclose the atomic construction of Huntington’s illness protein aggregates, providing insights into their position in neurodegeneration and doable treatments. (CREDIT: Zephyr/Science Supply) In spite of intensive analysis, the atomic-level construction of polyQ fibrils has remained elusive, essentially because of their repetitive sequences and distinct aggregation patterns. Those fibrils fluctuate from different amyloids through that includes antiparallel β-sheets and block-like cores, making them in particular difficult to check the use of conventional ways. Researchers have lengthy sought to conquer those obstacles to release insights crucial to working out and treating HD.To handle those demanding situations, a staff of world researchers led through Markus Miettinen on the College of Bergen hired integrative structural biology. Via combining information from ssNMR, cryo-EM, and complex computational modeling, they reconstructed the atomic-resolution construction of polyQ and HTTex1 fibrils. This interdisciplinary method bridges experimental and theoretical strategies, providing a complete working out of those distinctive protein clumps.The analysis published that the polyQ fibril core is composed of stacked β-sheets, each and every shaped through antiparallel β-strands with minimum turns. Those sheets are tightly packed, devoid of water, and showcase a repetitive, pseudo-symmetric association. Not like different amyloid fibrils, polyQ buildings lack the twisting patterns frequently noticed in parallel in-register β-sheets. As a substitute, their structure is characterised through “glutamine zippers” and hydrogen-bonded ladders, which stabilize the fibril core.The usage of molecular dynamics simulations and ssNMR information, researchers recognized two dominant glutamine conformations inside the fibril core. Those conformations, known as “a” and “b,” change in a repeating trend, growing a particular spectroscopic fingerprint. This fingerprint lets in for exact characterization of the fibril construction and gives doable goals for diagnostic imaging.The molecular modeling published that the polyQ core is shaped through an infinitely repeating eight-glutamine unit. This structural motif is notable for its translational symmetry and the absence of the twisting generally noticed in amyloid fibrils. Moreover, the construction fulfills crucial constraints required for steadiness, together with particular dihedral angles that allow interdigitating hydrogen bonds between aspect chains. Those findings underscore the individuality of HD-related fibrils and their deviation from typical amyloid buildings.Figuring out the construction of HTTex1 fibrils has vital implications for HD analysis. The molecular structure supplies insights into the organic houses of those fibrils, together with their resistance to degradation and their position in neurotoxicity. For example, the densely packed core and restricted solvent accessibility obstruct post-aggregation polyubiquitination, complicating the cell mechanisms for fibril clearance. Glutamine zippers and ladders inside the polyQ amyloid core. (CREDIT: Nature Communications) Additionally, the detailed structural information allow the design of focused ligands for imaging and diagnostics. Those ligands may just assist hit upon fibril formation in vivo, providing previous and extra correct diagnoses of HD. Moreover, the findings might information the advance of healing inhibitors or modulators to disrupt fibril formation and development.Markus Miettinen emphasised the significance of this analysis, declaring, “Figuring out the construction of protein clumps is a an important piece of the puzzle in working out how those proteins purpose illness. Our new molecular findings are crucial for additional creating diagnostic equipment and imaging ways to hit upon and track illness proteins in sufferers.”This groundbreaking learn about used to be made conceivable through a collaboration of researchers from establishments throughout Europe. Individuals incorporated Mahdi Bagherpoor Helabad from the Max Planck Institute of Colloids and Interfaces, Irina Matlahov and co-workers from the College of Groningen, and a number of other others. Their findings, revealed in Nature Communications, constitute a milestone in HD analysis and underscore the worth of interdisciplinary approaches in tackling advanced organic issues.Investment for this analysis got here from foundations supporting Huntington’s illness research, with vital contributions from households suffering from the illness. Their toughen highlights the crucial position of private and non-private partnerships in advancing medical discoveries.
Glutamine zippers and ladders inside the polyQ amyloid core. (CREDIT: Nature Communications) Additionally, the detailed structural information allow the design of focused ligands for imaging and diagnostics. Those ligands may just assist hit upon fibril formation in vivo, providing previous and extra correct diagnoses of HD. Moreover, the findings might information the advance of healing inhibitors or modulators to disrupt fibril formation and development.Markus Miettinen emphasised the significance of this analysis, declaring, “Figuring out the construction of protein clumps is a an important piece of the puzzle in working out how those proteins purpose illness. Our new molecular findings are crucial for additional creating diagnostic equipment and imaging ways to hit upon and track illness proteins in sufferers.”This groundbreaking learn about used to be made conceivable through a collaboration of researchers from establishments throughout Europe. Individuals incorporated Mahdi Bagherpoor Helabad from the Max Planck Institute of Colloids and Interfaces, Irina Matlahov and co-workers from the College of Groningen, and a number of other others. Their findings, revealed in Nature Communications, constitute a milestone in HD analysis and underscore the worth of interdisciplinary approaches in tackling advanced organic issues.Investment for this analysis got here from foundations supporting Huntington’s illness research, with vital contributions from households suffering from the illness. Their toughen highlights the crucial position of private and non-private partnerships in advancing medical discoveries. PolyQ peptide fibril construction. (CREDIT: Nature Communications) The learn about additionally highlights the wider applicability of integrative structural biology. Whilst the principle center of attention is on HD, the strategies hired may well be prolonged to different neurodegenerative sicknesses characterised through amyloid fibrils. Alzheimer’s and Parkinson’s sicknesses, as an example, contain protein aggregates with distinct buildings and houses. Via leveraging the equipment and methods advanced for learning polyQ fibrils, researchers might discover new healing goals throughout a spread of prerequisites.Whilst Huntington’s illness is the principle center of attention, the strategies and findings lengthen to different neurodegenerative sicknesses. Protein clumping is a not unusual function in problems like Alzheimer’s and Parkinson’s, despite the fact that the buildings fluctuate considerably. The original houses of HD-related fibrils lift intriguing questions on their formation mechanisms and organic roles.Along with providing hope for HD sufferers, the analysis contributes to the wider box of molecular biology. The equipment and methods advanced on this learn about are anticipated to facilitate additional breakthroughs in structural biology, paving the best way for cutting edge diagnostic and healing methods. For example, the usage of molecular simulations to enrich experimental information represents a vital development within the talent to fashion advanced organic methods correctly.Markus Miettinen highlighted the wider affect of this paintings, noting, “Past the brand new insights into Huntington’s illness, we’ve got advanced equipment that make molecular simulations extra obtainable to researchers international. This interdisciplinary method is the way forward for structural biology.”
PolyQ peptide fibril construction. (CREDIT: Nature Communications) The learn about additionally highlights the wider applicability of integrative structural biology. Whilst the principle center of attention is on HD, the strategies hired may well be prolonged to different neurodegenerative sicknesses characterised through amyloid fibrils. Alzheimer’s and Parkinson’s sicknesses, as an example, contain protein aggregates with distinct buildings and houses. Via leveraging the equipment and methods advanced for learning polyQ fibrils, researchers might discover new healing goals throughout a spread of prerequisites.Whilst Huntington’s illness is the principle center of attention, the strategies and findings lengthen to different neurodegenerative sicknesses. Protein clumping is a not unusual function in problems like Alzheimer’s and Parkinson’s, despite the fact that the buildings fluctuate considerably. The original houses of HD-related fibrils lift intriguing questions on their formation mechanisms and organic roles.Along with providing hope for HD sufferers, the analysis contributes to the wider box of molecular biology. The equipment and methods advanced on this learn about are anticipated to facilitate additional breakthroughs in structural biology, paving the best way for cutting edge diagnostic and healing methods. For example, the usage of molecular simulations to enrich experimental information represents a vital development within the talent to fashion advanced organic methods correctly.Markus Miettinen highlighted the wider affect of this paintings, noting, “Past the brand new insights into Huntington’s illness, we’ve got advanced equipment that make molecular simulations extra obtainable to researchers international. This interdisciplinary method is the way forward for structural biology.” Choose structural information on HTT Exon 1 aggregates. (CREDIT: Nature Communications) As analysis progresses, the working out of HD-related fibrils may additionally tell efforts to mitigate different amyloid-related sicknesses. The distinct structural options of polyQ fibrils, comparable to their block-like core and antiparallel β-sheet association, may just function a fashion for learning different ordinary amyloid buildings. The findings underscore the significance of persevered funding in fundamental and carried out analysis. Via addressing the molecular underpinnings of sicknesses like HD, scientists can increase focused methods to relieve struggling and enhance high quality of lifestyles for sufferers international. The collaborative nature of this paintings serves as a testomony to the ability of interdisciplinary science in overcoming advanced demanding situations.
Choose structural information on HTT Exon 1 aggregates. (CREDIT: Nature Communications) As analysis progresses, the working out of HD-related fibrils may additionally tell efforts to mitigate different amyloid-related sicknesses. The distinct structural options of polyQ fibrils, comparable to their block-like core and antiparallel β-sheet association, may just function a fashion for learning different ordinary amyloid buildings. The findings underscore the significance of persevered funding in fundamental and carried out analysis. Via addressing the molecular underpinnings of sicknesses like HD, scientists can increase focused methods to relieve struggling and enhance high quality of lifestyles for sufferers international. The collaborative nature of this paintings serves as a testomony to the ability of interdisciplinary science in overcoming advanced demanding situations.
Scientists make main new discovery in battle towards Huntington’s illness












