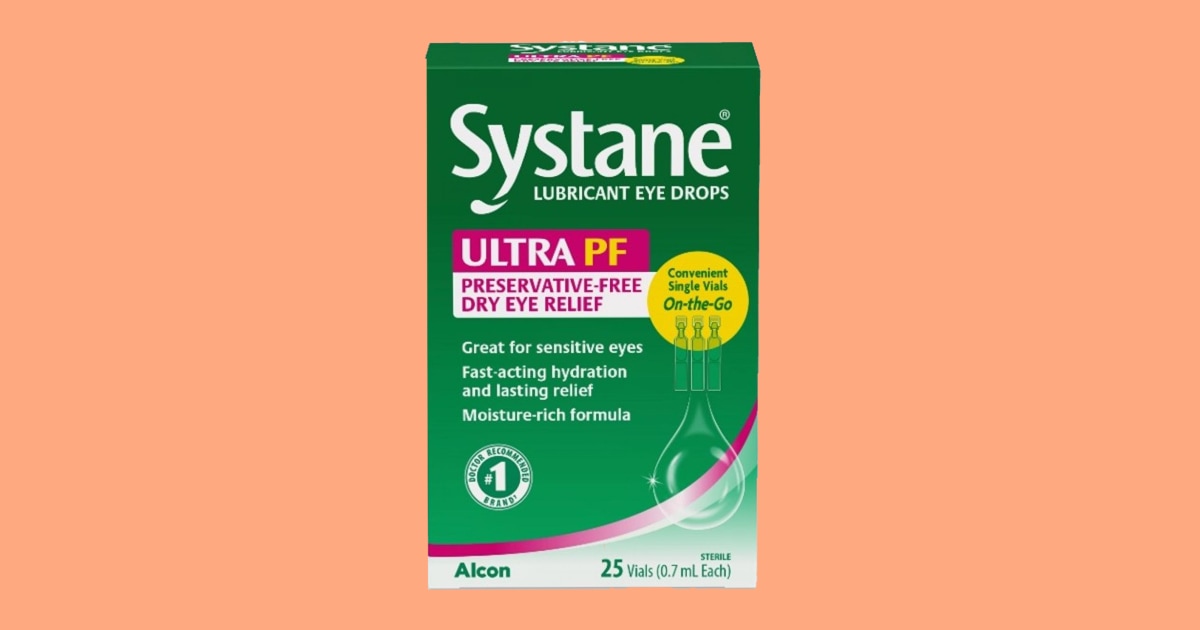
Systane emblem eye drops have been voluntarily recalled as a result of imaginable fungal contamination, the Meals and Drug Management mentioned. One lot of Systane Lubricant Eye Drops Extremely PF was once recalled following a shopper grievance “of overseas subject matter seen within a sealed single-use vial,” the FDA mentioned in a free up. It was once made up our minds that the fabric was once “fungal in nature.”The contamination may just doubtlessly purpose eye infections that “could also be vision-threatening, and in very uncommon circumstances doubtlessly life-threatening in immunocompromised sufferers,” consistent with the discharge. Alcon Laboratories, which producers the attention drops, has no longer won any studies of consumers struggling opposed results, the FDA mentioned. The recalled eye drops come within the 25-count on-the-go unmarried vials with lot quantity 10101 and an expiration date of September 2025. The product was once offered at Publix, consistent with a realize from the grocery chain.The drops are used to regard burning and inflammation in other people experiencing dry eye signs. Somebody who has bought the attention drops will have to forestall the use of them right away and will search both money back or alternative, the FDA mentioned. If somebody is experiencing signs, they will have to discuss with their doctor.Steven Smith, an Alcon spokesperson, mentioned that the corporate’s investigation is ongoing, however “the presence of overseas subject matter seems to be remoted to the only unit returned by way of a buyer.” Alcon recalled the product “out of an abundance of warning to prioritize client protection,” he added. Minyvonne BurkeMinyvonne Burke is a senior breaking information reporter for NBC Information. Insiya Gandhi contributed.
Systane eye drops recalled for imaginable fungal contamination, FDA says