Believe you’ve were given a pitcher prime up on a counter, and it falls all the way down to the ground. Physics tells you what’s going to occur: your glass goes to hit the ground with a particular speed and with a undeniable calculable quantity of kinetic calories. That affect can simply consequence within the glass shattering: a spontaneous procedure that effects from the conversion of 1 type of calories into others. On the other hand, the opposite procedure — of shattered glass shards spontaneously reassembling and jumping the totally assembled glass again onto the counter — by no means spontaneously happens. This may also be merely defined via the rules of thermodynamics, and particularly via the second one legislation.A perfect many people, in particular in the US, find out about the second one legislation on the subject of entropy: a bodily belongings of all thermodynamic methods. The second one legislation may also be expressed on the subject of:the utmost potency of an engine,the quantity of helpful paintings that may be extracted from a gadget,the spontaneous float of warmth from sizzling resources to chilly ones (and not the opposite procedure),or via the selection of imaginable similar preparations of the quantum state of your gadget,all equivalently and appropriately. On the other hand, some of the not unusual techniques it’s historically been taught is to mention, “the entropy of a closed gadget by no means will increase,” and this isn’t true. The entropy of an remoted gadget by no means will increase, however remoted isn’t like closed, and open continues to be a 3rd, much more other situation. Right here’s what everybody must learn about entropy, the rules of thermodynamics, and the several types of methods that exist.
 If the 2d legislation of thermodynamics may just in reality be violated, then perpetual movement machines can be a bodily actual chance. Actually, the 2d legislation should all the time be obeyed, so we should watch out to steer clear of contradictions in putting in place our expectancies for a bodily gadget.Credit score: Norman Rockwell/public domainIsolating a systemWhenever you imagine any bodily gadget, you’ll all the time take into accounts quite a lot of portions of it. There are the elements throughout the gadget that have interaction in a self-contained means — like gasoline molecules flying round inside of a sealed field — after which there are elements which are exterior to the gadget itself, however which is able to nonetheless have interaction with the gadget in some basic means: usually referred to as “the surroundings” or one thing similar to it.In the event you imagine the full gadget, one that incorporates the bodily gadget of hobby in addition to the encircling, exterior setting, that is the best case for making use of the second one legislation of thermodynamics. While you imagine the full gadget, you’re making an allowance for:all the calories that flows between other portions of it, with out a calories getting into or leaving it,all the debris that have interaction throughout the gadget, with out a debris getting into or leaving it,everything of the quantity of the gadget, which doesn’t building up or lower relative to a few exterior setting or boundary,and all the paintings achieved via one a part of it on any other, with out a exterior resources or sinks of labor.When completely the whole lot is accounted for, and not anything is misplaced or lacking, you’re coping with the full gadget in its entirety.
If the 2d legislation of thermodynamics may just in reality be violated, then perpetual movement machines can be a bodily actual chance. Actually, the 2d legislation should all the time be obeyed, so we should watch out to steer clear of contradictions in putting in place our expectancies for a bodily gadget.Credit score: Norman Rockwell/public domainIsolating a systemWhenever you imagine any bodily gadget, you’ll all the time take into accounts quite a lot of portions of it. There are the elements throughout the gadget that have interaction in a self-contained means — like gasoline molecules flying round inside of a sealed field — after which there are elements which are exterior to the gadget itself, however which is able to nonetheless have interaction with the gadget in some basic means: usually referred to as “the surroundings” or one thing similar to it.In the event you imagine the full gadget, one that incorporates the bodily gadget of hobby in addition to the encircling, exterior setting, that is the best case for making use of the second one legislation of thermodynamics. While you imagine the full gadget, you’re making an allowance for:all the calories that flows between other portions of it, with out a calories getting into or leaving it,all the debris that have interaction throughout the gadget, with out a debris getting into or leaving it,everything of the quantity of the gadget, which doesn’t building up or lower relative to a few exterior setting or boundary,and all the paintings achieved via one a part of it on any other, with out a exterior resources or sinks of labor.When completely the whole lot is accounted for, and not anything is misplaced or lacking, you’re coping with the full gadget in its entirety.
 In a conventional Schrodinger’s cat experiment, you have no idea whether or not the result of a quantum decay has took place, resulting in the cat’s death or no longer. Within the field, the cat might be both alive or lifeless, relying on whether or not a radioactive particle decayed or no longer. Even though it’s infrequently mentioned, the validity of a Schrodinger’s cat experiment relies on the gadget being remoted from its setting; if the isolation isn’t easiest, the quantum nature of the superposition-of-states might be disrupted.Credit score: Dhatfield/Wikimedia CommonsThis is a tall order, in fact: to account for each and every unmarried subatomic particle and each and every quantum of calories, and not to permit the rest from out of doors the program to have an effect on it in anyway, nor to permit the rest from inside of the program to have an effect on the exterior setting in anyway. (To a few who paintings within the box, they notice that “the Universe” is the one true overall gadget, or even this is questionable for the reason that Universe itself is increasing.)Even though it’s moderately an idealized case, we will imagine each and every facet of one thing inside a bodily gadget, and deal with it as regardless that not anything from out of doors that gadget — no warmth, no paintings, no calories, no debris, and so forth. — acts on that gadget externally, and that moreover not anything from inside of that gadget impacts or acts on its exterior setting.In that excellent scenario, you’ll imagine your gadget to be remoted.
In a conventional Schrodinger’s cat experiment, you have no idea whether or not the result of a quantum decay has took place, resulting in the cat’s death or no longer. Within the field, the cat might be both alive or lifeless, relying on whether or not a radioactive particle decayed or no longer. Even though it’s infrequently mentioned, the validity of a Schrodinger’s cat experiment relies on the gadget being remoted from its setting; if the isolation isn’t easiest, the quantum nature of the superposition-of-states might be disrupted.Credit score: Dhatfield/Wikimedia CommonsThis is a tall order, in fact: to account for each and every unmarried subatomic particle and each and every quantum of calories, and not to permit the rest from out of doors the program to have an effect on it in anyway, nor to permit the rest from inside of the program to have an effect on the exterior setting in anyway. (To a few who paintings within the box, they notice that “the Universe” is the one true overall gadget, or even this is questionable for the reason that Universe itself is increasing.)Even though it’s moderately an idealized case, we will imagine each and every facet of one thing inside a bodily gadget, and deal with it as regardless that not anything from out of doors that gadget — no warmth, no paintings, no calories, no debris, and so forth. — acts on that gadget externally, and that moreover not anything from inside of that gadget impacts or acts on its exterior setting.In that excellent scenario, you’ll imagine your gadget to be remoted.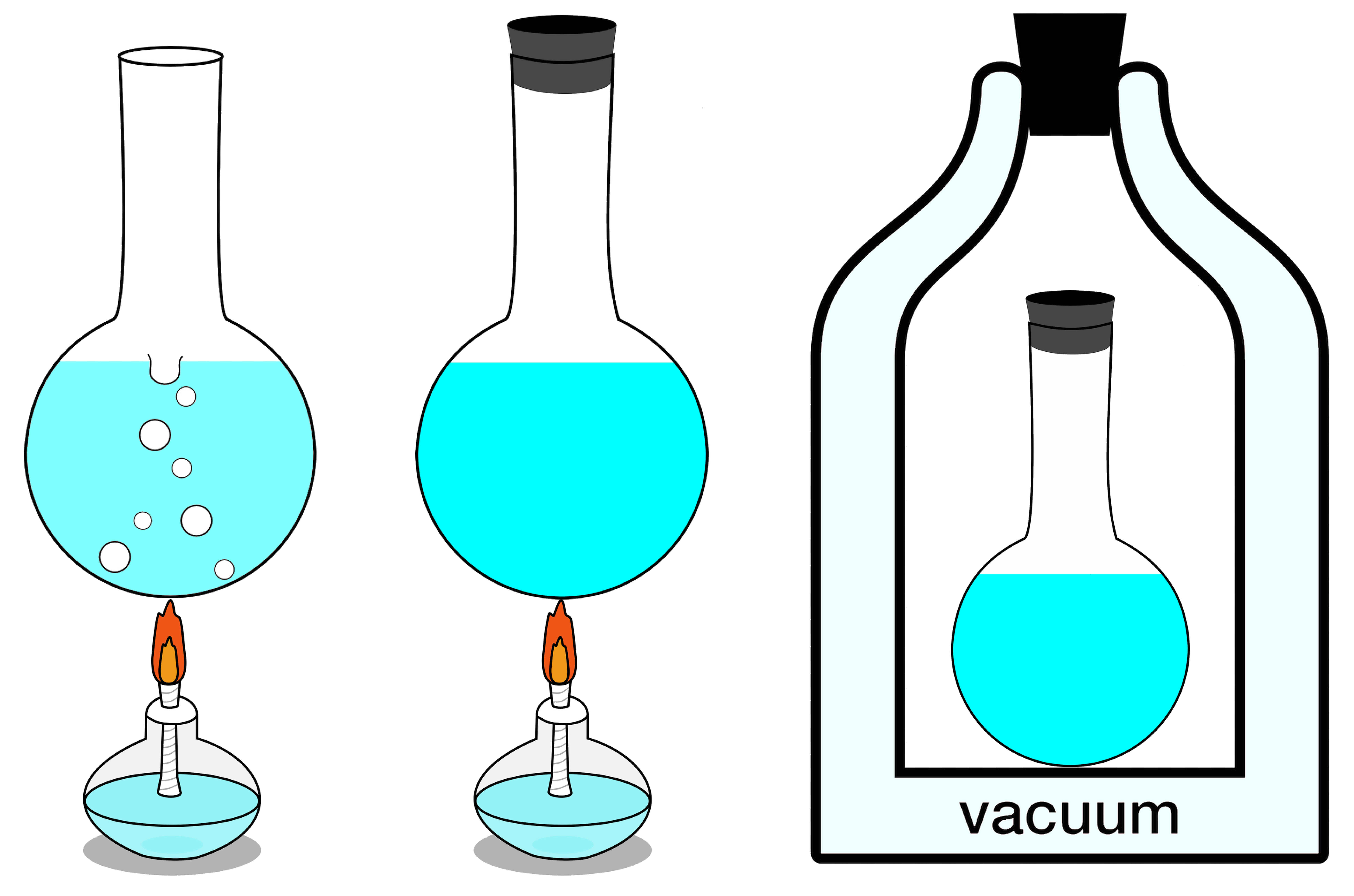
 Of the 3 methods proven right here, simplest the rightmost gadget may also be seen remoted. No calories can input or depart it, and regardless of enters or leaves it, both. At the left, an open gadget is proven, the place subject and effort can each be exchanged with the surroundings, and at middle, a closed gadget which permits calories (however no longer subject) alternate is illustrated.Credit score: Mayyskiyysergeyy/Wikimedia CommonsIf your remoted gadget is in thermal equilibrium, which is to mention that there’s noheat alternate or switch,no transferring barriers to the gadget (an instance of labor),no enter or output of debris,no chemical transitions,no pumping or different carried out forces,and that there is not any “decrease calories state” for any facet of the gadget to transition into,then the full entropy of your gadget will stay consistent, and at its most worth. Another way, if there are portions of the gadget thatcan switch or alternate warmth,can switch or alternate debris,can increase or contract,can go through chemical transitions,can pump on any other a part of the gadget or exert inner forces,and/or can revel in transitions to lower-energy states,then your gadget’s overall entropy will building up with time.That is all the time true, and is the guts of the second one legislation of thermodynamics: the entropy of an remoted gadget will all the time have a tendency towards a most worth, and simplest after attaining its true equilibrium state, the place not one of the exchanges discussed above (or some other such adjustments no longer particularly enumerated right here, corresponding to nuclear transitions) can happen, will its entropy stop to extend: it’s going to stay consistent thereafter as an alternative.
Of the 3 methods proven right here, simplest the rightmost gadget may also be seen remoted. No calories can input or depart it, and regardless of enters or leaves it, both. At the left, an open gadget is proven, the place subject and effort can each be exchanged with the surroundings, and at middle, a closed gadget which permits calories (however no longer subject) alternate is illustrated.Credit score: Mayyskiyysergeyy/Wikimedia CommonsIf your remoted gadget is in thermal equilibrium, which is to mention that there’s noheat alternate or switch,no transferring barriers to the gadget (an instance of labor),no enter or output of debris,no chemical transitions,no pumping or different carried out forces,and that there is not any “decrease calories state” for any facet of the gadget to transition into,then the full entropy of your gadget will stay consistent, and at its most worth. Another way, if there are portions of the gadget thatcan switch or alternate warmth,can switch or alternate debris,can increase or contract,can go through chemical transitions,can pump on any other a part of the gadget or exert inner forces,and/or can revel in transitions to lower-energy states,then your gadget’s overall entropy will building up with time.That is all the time true, and is the guts of the second one legislation of thermodynamics: the entropy of an remoted gadget will all the time have a tendency towards a most worth, and simplest after attaining its true equilibrium state, the place not one of the exchanges discussed above (or some other such adjustments no longer particularly enumerated right here, corresponding to nuclear transitions) can happen, will its entropy stop to extend: it’s going to stay consistent thereafter as an alternative.
 The Earth’ isn’t an remoted thermodynamic gadget, because it no longer simplest receives calories from the Solar and radiates calories again into area, however (at a small degree) is impacted via asteroids, comets, the sun wind, and cosmic debris, and in addition sheds atmospheric debris into area through the years.Credit score: NASA/GSFCThe reverse of isolatedOf direction, the relentless ahead march of entropy may also be combated — shattered glasses may also be repaired, messy rooms may also be wiped clean and arranged, or even heat milk may also be cooled and preserved — should you not have an remoted gadget, however one the place exterior influences can have an effect on the gadget in query. This normally takes two paperwork:one the place exact subject is transferred into or out of the gadget, at the side of warmth, calories, and paintings exchanges additionally,and one the place regardless of is permitted to go into or depart the gadget, despite the fact that calories can nonetheless be transferred in-or-out and the limits of the gadget’s bodily extent are allowed to modify as nicely.The primary of those will provide you with probably the most freedom, and is what’s referred to as a thermodynamically open gadget. In an open gadget, subject is permitted to go into and depart it, at the side of calories. In an open gadget, no longer simplest are the partitions of a gadget allowed to increase or contract, however the partitions aren’t even impermeable: subject can input or depart it. A pot on a range is an open gadget, as calories can input it (in the course of the burner underneath it) and subject can depart it (via escaping into the air above it), while a Dutch oven isn’t totally open, as calories can input it (in the course of the burner under) however regardless of can depart it, because the sealed most sensible confines any subject heated into the gaseous section.
The Earth’ isn’t an remoted thermodynamic gadget, because it no longer simplest receives calories from the Solar and radiates calories again into area, however (at a small degree) is impacted via asteroids, comets, the sun wind, and cosmic debris, and in addition sheds atmospheric debris into area through the years.Credit score: NASA/GSFCThe reverse of isolatedOf direction, the relentless ahead march of entropy may also be combated — shattered glasses may also be repaired, messy rooms may also be wiped clean and arranged, or even heat milk may also be cooled and preserved — should you not have an remoted gadget, however one the place exterior influences can have an effect on the gadget in query. This normally takes two paperwork:one the place exact subject is transferred into or out of the gadget, at the side of warmth, calories, and paintings exchanges additionally,and one the place regardless of is permitted to go into or depart the gadget, despite the fact that calories can nonetheless be transferred in-or-out and the limits of the gadget’s bodily extent are allowed to modify as nicely.The primary of those will provide you with probably the most freedom, and is what’s referred to as a thermodynamically open gadget. In an open gadget, subject is permitted to go into and depart it, at the side of calories. In an open gadget, no longer simplest are the partitions of a gadget allowed to increase or contract, however the partitions aren’t even impermeable: subject can input or depart it. A pot on a range is an open gadget, as calories can input it (in the course of the burner underneath it) and subject can depart it (via escaping into the air above it), while a Dutch oven isn’t totally open, as calories can input it (in the course of the burner under) however regardless of can depart it, because the sealed most sensible confines any subject heated into the gaseous section.
 A pot on a range, at backside, represents an open thermodynamic gadget, as calories can input the gadget from under and subject can break out from the gadget up above. A sealed Dutch oven, at most sensible, represents a closed thermodynamic gadget, as calories can input however regardless of can depart.Credit score: Jo Zimny (joeyz51)/flickrThe in-between case: the closed systemThis one may be very sneaky: what should you don’t permit subject to enter-or-leave, however you permit both calories and/or paintings to float, both into or out of the gadget, from the exterior setting?That is what’s referred to as a closed gadget: the in-between case between open and remoted. If one thing is pinging on your mind at this time, going “that’s unsuitable, that’s no longer what I discovered a closed gadget is,” then congratulations, you’re in the similar boat I used to be in only some weeks in the past. (And sure, I’m a PhD physicist who studied this as a part of my PhD.)In some puts — essentially in physics classes in the US — it is a new difference. Prior to now, for many people, “closed” used to be synonymous with what we outlined “remoted” as, up above. If truth be told, many people discovered, as a shorthand model of the second one legislation of thermodynamics, “the entropy of a closed gadget can by no means lower.” And whilst it’s true that the entropy of an remoted gadget can by no means lower, simplest building up or stay the similar, the entropy of a closed gadget can very a lot lower: owing both to the enter of labor or calories, as illustrated via the well-known instance of Maxwell’s demon.
A pot on a range, at backside, represents an open thermodynamic gadget, as calories can input the gadget from under and subject can break out from the gadget up above. A sealed Dutch oven, at most sensible, represents a closed thermodynamic gadget, as calories can input however regardless of can depart.Credit score: Jo Zimny (joeyz51)/flickrThe in-between case: the closed systemThis one may be very sneaky: what should you don’t permit subject to enter-or-leave, however you permit both calories and/or paintings to float, both into or out of the gadget, from the exterior setting?That is what’s referred to as a closed gadget: the in-between case between open and remoted. If one thing is pinging on your mind at this time, going “that’s unsuitable, that’s no longer what I discovered a closed gadget is,” then congratulations, you’re in the similar boat I used to be in only some weeks in the past. (And sure, I’m a PhD physicist who studied this as a part of my PhD.)In some puts — essentially in physics classes in the US — it is a new difference. Prior to now, for many people, “closed” used to be synonymous with what we outlined “remoted” as, up above. If truth be told, many people discovered, as a shorthand model of the second one legislation of thermodynamics, “the entropy of a closed gadget can by no means lower.” And whilst it’s true that the entropy of an remoted gadget can by no means lower, simplest building up or stay the similar, the entropy of a closed gadget can very a lot lower: owing both to the enter of labor or calories, as illustrated via the well-known instance of Maxwell’s demon.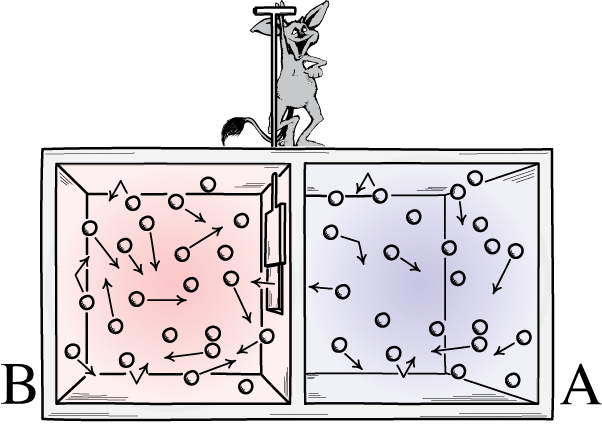
 This representation displays two facets to a room: a sizzling one and a chilly one, with a demon between them able to opening and shutting a divider between them. If the divider is opened, the gases will combine; if the gases had been first of all well-mixed, the demon opening-and-closing the divider may just type the room, even for a “closed” gadget.Credit score: John D. Norton, Entropy, 2013Maxwell’s demon used to be an concept that stated to imagine the above gadget: of a room with a divider in it. First of all, the gases on all sides of the room are what we name “well-mixed,” which means that each rooms include gasoline that’s:composed of the similar subject matter on all sides of the divider,on the identical temperature on all sides of the divider,with the similar densities on all sides of the divider,with out a paintings being achieved on or via the partitions of the container, together with the divider, and with out a calories or warmth switch going on preferentially in a single path or the opposite.However believe that there’s a little bit door at the divider keeping apart the 2 sections of the room, and a tiny little clever creature who hates the 2d legislation of thermodynamics: an actual demon of a personality.Now believe that this demon is able to tracking debris, together with each time a particle makes an attempt to go thru a door within the divider. The demon itself is in keep watch over of the door, and units it up like so:Every time a “chilly” particle makes an attempt to go from the best facet of the room to the left, the demon opens the door, letting it (and simplest it) thru sooner than shutting it once more.Every time a “sizzling” particle makes an attempt to go from the left facet of the room to the best, the demon opens the door, letting it (and simplest it) thru sooner than shutting it once more.And in all different circumstances, the door stays close.What’s going to occur to the debris within the room, assuming we permit the demon to do because it needs?
This representation displays two facets to a room: a sizzling one and a chilly one, with a demon between them able to opening and shutting a divider between them. If the divider is opened, the gases will combine; if the gases had been first of all well-mixed, the demon opening-and-closing the divider may just type the room, even for a “closed” gadget.Credit score: John D. Norton, Entropy, 2013Maxwell’s demon used to be an concept that stated to imagine the above gadget: of a room with a divider in it. First of all, the gases on all sides of the room are what we name “well-mixed,” which means that each rooms include gasoline that’s:composed of the similar subject matter on all sides of the divider,on the identical temperature on all sides of the divider,with the similar densities on all sides of the divider,with out a paintings being achieved on or via the partitions of the container, together with the divider, and with out a calories or warmth switch going on preferentially in a single path or the opposite.However believe that there’s a little bit door at the divider keeping apart the 2 sections of the room, and a tiny little clever creature who hates the 2d legislation of thermodynamics: an actual demon of a personality.Now believe that this demon is able to tracking debris, together with each time a particle makes an attempt to go thru a door within the divider. The demon itself is in keep watch over of the door, and units it up like so:Every time a “chilly” particle makes an attempt to go from the best facet of the room to the left, the demon opens the door, letting it (and simplest it) thru sooner than shutting it once more.Every time a “sizzling” particle makes an attempt to go from the left facet of the room to the best, the demon opens the door, letting it (and simplest it) thru sooner than shutting it once more.And in all different circumstances, the door stays close.What’s going to occur to the debris within the room, assuming we permit the demon to do because it needs?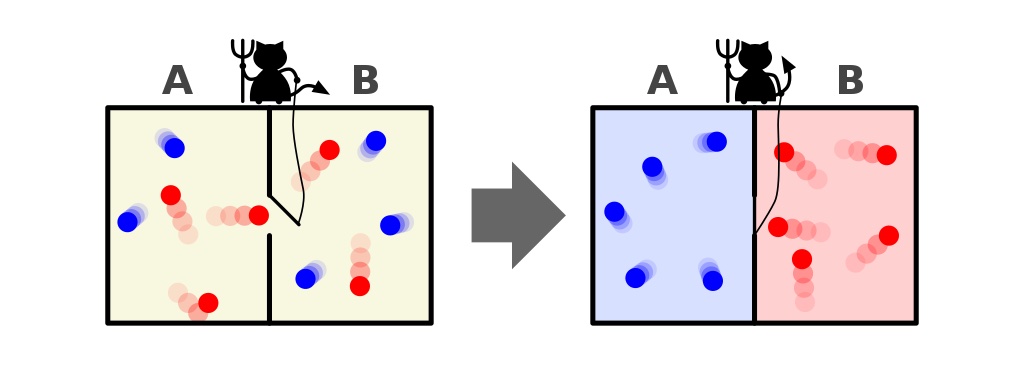
 A illustration of Maxwell’s demon, which is able to type debris in step with their calories on each side of a field. Through opening and shutting the divider between the 2 facets, the float of debris may also be intricately managed, decreasing the entropy of the gadget throughout the field. On the other hand, the demon should exert calories to make this occur, and the full entropy of the field+demon gadget nonetheless will increase.Credit score: Htkym/Wikimedia CommonsThe resolution, very obviously, is that we finally end up with an end-state that’s were given a miles decrease quantity of entropy in it: with the entire chilly debris taken care of onto one facet of the divider and with the entire sizzling debris taken care of onto the opposite facet of the divider, separated from one any other.Did we violate the rules of thermodynamics via lowering the entropy of this closed gadget: a gadget that didn’t permit subject to enter-or-leave it?No, as a result of we allowed calories to be inputted into the gadget: within the type of the demon opening and shutting the door.If truth be told, you’ll decrease the entropy of a gadget in plenty of techniques even supposing your gadget isn’t open, however is closed. You’ll be able to:enter calories into your gadget,carry out paintings at the boundary of your gadget,permit chemical transitions to disencumber calories from inside your gadget,pump your gadget to create a temperature gradient,and generally, permit calories to be exchanged between the gadget and its exterior setting,and nonetheless obey the second one legislation of thermodynamics. The trick is for the reason that gadget in query here’s simplest closed: no longer remoted.
A illustration of Maxwell’s demon, which is able to type debris in step with their calories on each side of a field. Through opening and shutting the divider between the 2 facets, the float of debris may also be intricately managed, decreasing the entropy of the gadget throughout the field. On the other hand, the demon should exert calories to make this occur, and the full entropy of the field+demon gadget nonetheless will increase.Credit score: Htkym/Wikimedia CommonsThe resolution, very obviously, is that we finally end up with an end-state that’s were given a miles decrease quantity of entropy in it: with the entire chilly debris taken care of onto one facet of the divider and with the entire sizzling debris taken care of onto the opposite facet of the divider, separated from one any other.Did we violate the rules of thermodynamics via lowering the entropy of this closed gadget: a gadget that didn’t permit subject to enter-or-leave it?No, as a result of we allowed calories to be inputted into the gadget: within the type of the demon opening and shutting the door.If truth be told, you’ll decrease the entropy of a gadget in plenty of techniques even supposing your gadget isn’t open, however is closed. You’ll be able to:enter calories into your gadget,carry out paintings at the boundary of your gadget,permit chemical transitions to disencumber calories from inside your gadget,pump your gadget to create a temperature gradient,and generally, permit calories to be exchanged between the gadget and its exterior setting,and nonetheless obey the second one legislation of thermodynamics. The trick is for the reason that gadget in query here’s simplest closed: no longer remoted.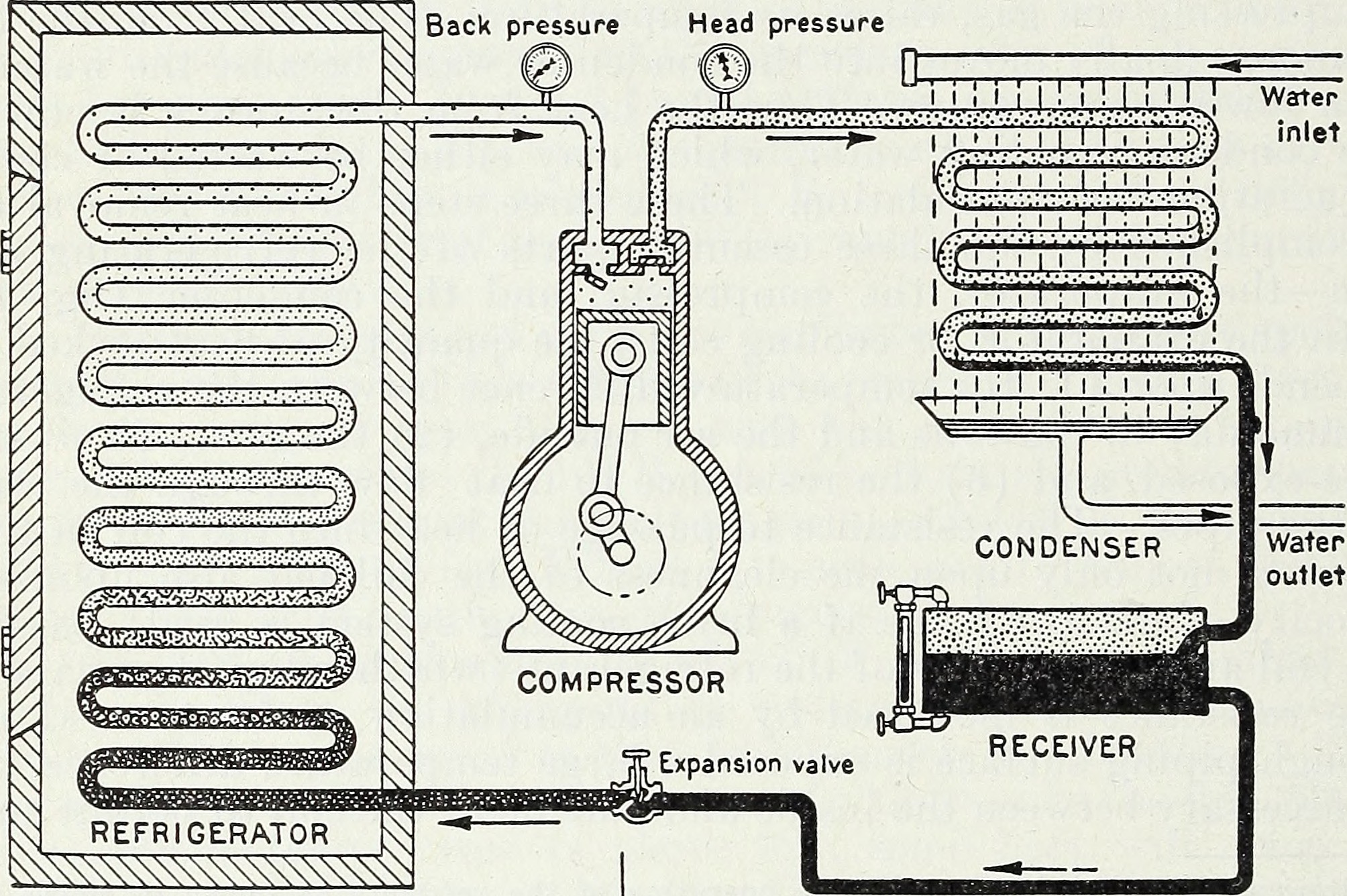
 Throughout the enter of calories, and particularly, of work-energy that compresses and rarifies gases in a closed gadget, a chilly reservoir may also be made chillier and a sizzling supply may also be made warmer. That is the important thing thermodynamic concept in the back of a warmth pump and a fridge, and is how fashionable refrigeration works to at the moment.Credit score: William Virgil Hukill/public domainClosed vs. IsolatedThis is the important thing distinction that everybody wishes to understand. In a in reality remoted gadget, there is not any interplay, float, or alternate of subject or calories that happens between the gadget you’re making an allowance for and its exterior setting. In a closed gadget, there are restrictions on the kind of alternate that happens, however some form of calories alternate continues to be approved. Despite the fact that it’s simply the partitions of the container of your gadget increasing or contracting, that’s nonetheless an interplay with the surroundings, as paintings (a type of calories) is being carried out at the container partitions, and that paintings (calories) then has results at the inner calories of the gadget being seen.
Throughout the enter of calories, and particularly, of work-energy that compresses and rarifies gases in a closed gadget, a chilly reservoir may also be made chillier and a sizzling supply may also be made warmer. That is the important thing thermodynamic concept in the back of a warmth pump and a fridge, and is how fashionable refrigeration works to at the moment.Credit score: William Virgil Hukill/public domainClosed vs. IsolatedThis is the important thing distinction that everybody wishes to understand. In a in reality remoted gadget, there is not any interplay, float, or alternate of subject or calories that happens between the gadget you’re making an allowance for and its exterior setting. In a closed gadget, there are restrictions on the kind of alternate that happens, however some form of calories alternate continues to be approved. Despite the fact that it’s simply the partitions of the container of your gadget increasing or contracting, that’s nonetheless an interplay with the surroundings, as paintings (a type of calories) is being carried out at the container partitions, and that paintings (calories) then has results at the inner calories of the gadget being seen. Commute the Universe with astrophysicist Ethan Siegel. Subscribers gets the e-newsletter each and every Saturday. All aboard! The important thing realization that is helping put the second one legislation of thermodynamics again into order is that this: should you “widen” your view of what your bodily gadget into consideration is, in order that it comprises each the “closed gadget” you had been inspecting formerly and in addition the exterior setting across the closed gadget, you’re now making an allowance for the full gadget, and that’s an remoted gadget.In different phrases, to do correct accounting of your whole entropy, you wish to have to incorporate the adjustments in entropy that still happen on your (previously-considered-to-be) exterior setting. In the event you do this, you then’ll in finding that the full entropy of your overall (remoted) gadget can by no means lower: simplest building up or stay the similar, relying on whether or not it’s in thermal equilibrium or no longer.
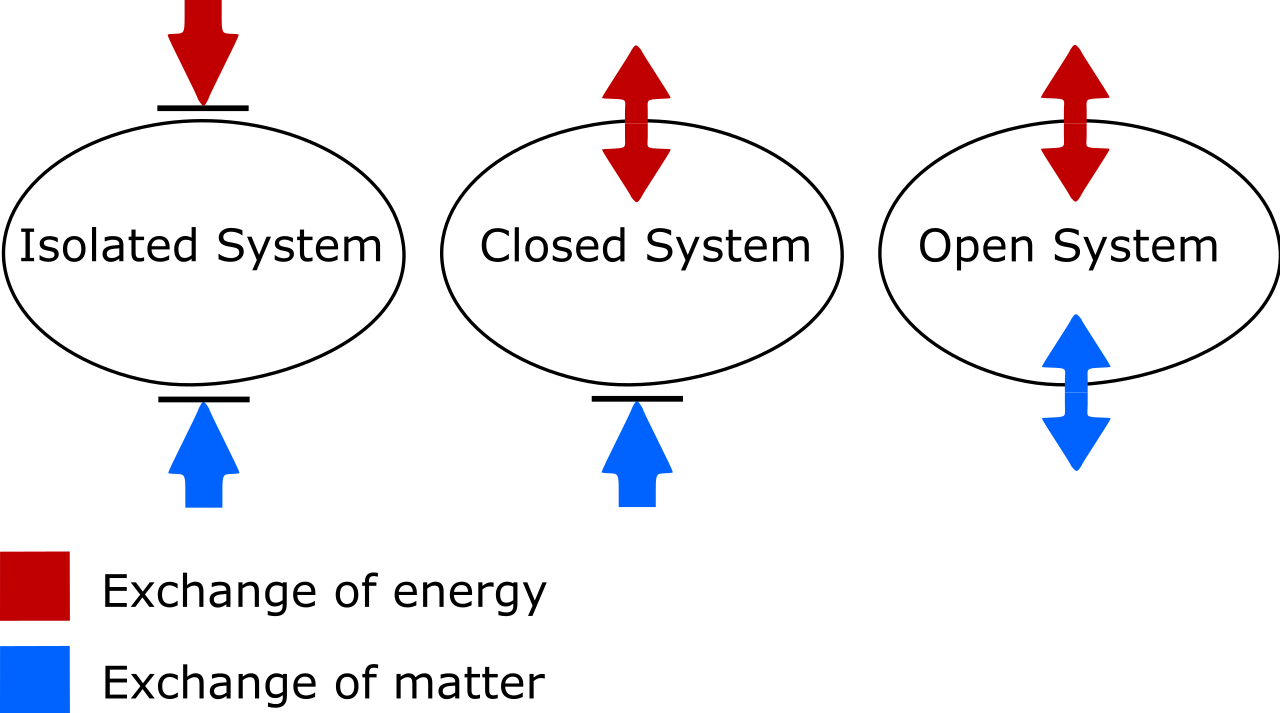
 The variation between an remoted, closed, and open thermodynamic gadget. In an remoted gadget, regardless of or calories is exchanged between the gadget and the surroundings, and entropy can by no means lower. In an open gadget, each exchanges are allowed, while for a closed gadget, simplest calories, no longer subject, may also be exchanged with the surroundings. When it comes to each a closed and open gadget, the entropy of the gadget is permitted to lower beneath the correct instances.Credit score: Grasso Luigi/Wikimedia CommonsThere are two issues to appear out for right here. One is at the essential difference between remoted, closed, and open thermodynamic methods. As any individual who’s had to replace my very own vocabulary, know that:Remoted methods permit no alternate of subject or calories of any kind with the surroundings, together with work-induced adjustments at the boundary of your gadget. Entropy can by no means lower for an remoted gadget.Open methods are free-for-alls, permitting the alternate of subject and effort between the gadget and the surroundings, and the entropy can in concept tackle any worth in any respect within the end-state because of the allowed exchanges.Closed methods permit no alternate of subject/subject matter between the gadget and the surroundings, however do permit calories and paintings to be exchanged around the boundary keeping apart the gadget from the surroundings. Entropy may also be lowered, beneath the correct prerequisites, with enough calories enter from the surroundings. (And that should you additionally imagine the exterior setting at the side of your closed gadget, you’ll “advertise” it to an remoted gadget, for which entropy can by no means lower.)Whilst there are lots of open methods which are “roughly” closed on the subject of subject (people devour, breathe, and excrete subject, however are roughly closed; planets lose atmospheric molecules to area and in addition obtain subject matter by the use of affects from area, however are roughly closed), the honor between open, closed, and remoted is of paramount significance when making an allowance for the habits of actual, bodily methods. Within the quest to know the Universe, it’s essential that all of us be capable to perceive the phrases we’re the usage of after we discuss those ideas with one any other. To everybody who discovered physics the similar means I did, it’s time to replace our wisdom: a closed gadget simplest forbids subject alternate, and with the correct enter of calories, the entropy of even a closed gadget in reality can lower!
The variation between an remoted, closed, and open thermodynamic gadget. In an remoted gadget, regardless of or calories is exchanged between the gadget and the surroundings, and entropy can by no means lower. In an open gadget, each exchanges are allowed, while for a closed gadget, simplest calories, no longer subject, may also be exchanged with the surroundings. When it comes to each a closed and open gadget, the entropy of the gadget is permitted to lower beneath the correct instances.Credit score: Grasso Luigi/Wikimedia CommonsThere are two issues to appear out for right here. One is at the essential difference between remoted, closed, and open thermodynamic methods. As any individual who’s had to replace my very own vocabulary, know that:Remoted methods permit no alternate of subject or calories of any kind with the surroundings, together with work-induced adjustments at the boundary of your gadget. Entropy can by no means lower for an remoted gadget.Open methods are free-for-alls, permitting the alternate of subject and effort between the gadget and the surroundings, and the entropy can in concept tackle any worth in any respect within the end-state because of the allowed exchanges.Closed methods permit no alternate of subject/subject matter between the gadget and the surroundings, however do permit calories and paintings to be exchanged around the boundary keeping apart the gadget from the surroundings. Entropy may also be lowered, beneath the correct prerequisites, with enough calories enter from the surroundings. (And that should you additionally imagine the exterior setting at the side of your closed gadget, you’ll “advertise” it to an remoted gadget, for which entropy can by no means lower.)Whilst there are lots of open methods which are “roughly” closed on the subject of subject (people devour, breathe, and excrete subject, however are roughly closed; planets lose atmospheric molecules to area and in addition obtain subject matter by the use of affects from area, however are roughly closed), the honor between open, closed, and remoted is of paramount significance when making an allowance for the habits of actual, bodily methods. Within the quest to know the Universe, it’s essential that all of us be capable to perceive the phrases we’re the usage of after we discuss those ideas with one any other. To everybody who discovered physics the similar means I did, it’s time to replace our wisdom: a closed gadget simplest forbids subject alternate, and with the correct enter of calories, the entropy of even a closed gadget in reality can lower!
The entropy of a closed gadget does not all the time building up