Given the correct prerequisites, tiny airborne debris of the rock mineral feldspar can affect cloud formation. Simply how this occurs, on the other hand, hasn’t ever been transparent.Now new analysis has now solved the thriller of ways this procedure in reality works.It is in the past been established that this ubiquitous subject matter – which makes up part of Earth’s crust and may also be discovered on different planets – is sexy to water molecules, making it a just right nucleation seed for vapor.As water molecules connect to feldspar mud excessive within the setting and get started freezing, the seeds of clouds are sown. On this new analysis, a group from the Vienna College of Generation (TU Wein) in Austria used a extremely delicate atomic power microscope to take a more in-depth glance.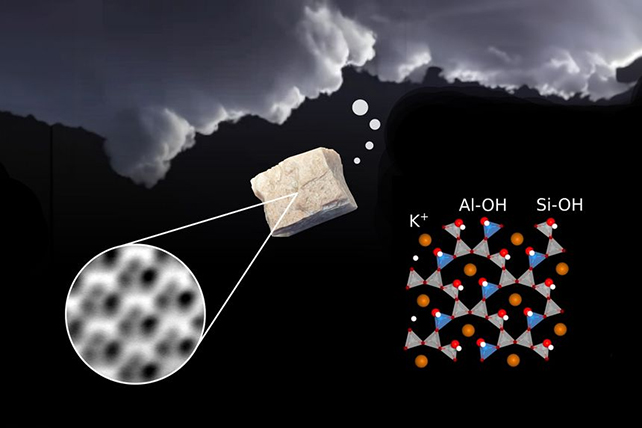 Scientists took an excessively shut have a look at feldspar. (TU Wein)”We positioned a work of feldspar within the microscope’s vacuum chamber and break up it in part to acquire a pristine and blank floor,” says TU Wein physicist Giada Franceschi. “We had been confused through the effects: the pictures of the outside regarded other from what not unusual theories had predicted.”The ordinary geometry of the feldspar floor printed through the ultra-high answer photographs used to be discovered to be brought about through tiny wallet of water known as inclusions. It seems that after the rock will get break up, a small quantity of water vapor will get launched from those wallet, which then attaches again to the outside.This attachment and the power launched through the splitting of the rock reasons the water molecules themselves to damage aside, growing what are referred to as hydroxyl teams (OH) – unmarried oxygen and hydrogen atoms related in combination.And it is those hydroxyl teams which can be key to the shut enchantment between water and feldspar: because the researchers had been in a position to verify through operating pc simulations of the chemical reactions, the hydroxyl teams are easiest anchor issues for water molecules to glue themselves to.”The bond is established very simply and briefly, and additionally it is very strong,” says physicist Ulrike Diebold, from TU Wein.”To take away the hydroxyl layer from feldspar, one must warmth it to a excessive temperature.”Feldspar is the most important subject matter for Earth’s carbon and potassium cycles in addition to the water cycle, and figuring out extra about the way it interacts with different components will train us extra about the ones cycles too.In the case of cloud formation, it is important that we know how local weather exchange goes to impact the ambience and the clouds in it – every other space the place this find out about will turn out helpful at some point.For now, it solves probably the most mysteries of feldspar that had researchers confused. Earlier hypotheses had thought to be the results of the potassium atoms within the rock, and the defects in its crystal construction.”Researchers had been making an allowance for a number of concepts why feldspar is such an efficient nucleation seed,” says Diebold.The analysis has been printed within the Magazine of Bodily Chemistry Letters.
Scientists took an excessively shut have a look at feldspar. (TU Wein)”We positioned a work of feldspar within the microscope’s vacuum chamber and break up it in part to acquire a pristine and blank floor,” says TU Wein physicist Giada Franceschi. “We had been confused through the effects: the pictures of the outside regarded other from what not unusual theories had predicted.”The ordinary geometry of the feldspar floor printed through the ultra-high answer photographs used to be discovered to be brought about through tiny wallet of water known as inclusions. It seems that after the rock will get break up, a small quantity of water vapor will get launched from those wallet, which then attaches again to the outside.This attachment and the power launched through the splitting of the rock reasons the water molecules themselves to damage aside, growing what are referred to as hydroxyl teams (OH) – unmarried oxygen and hydrogen atoms related in combination.And it is those hydroxyl teams which can be key to the shut enchantment between water and feldspar: because the researchers had been in a position to verify through operating pc simulations of the chemical reactions, the hydroxyl teams are easiest anchor issues for water molecules to glue themselves to.”The bond is established very simply and briefly, and additionally it is very strong,” says physicist Ulrike Diebold, from TU Wein.”To take away the hydroxyl layer from feldspar, one must warmth it to a excessive temperature.”Feldspar is the most important subject matter for Earth’s carbon and potassium cycles in addition to the water cycle, and figuring out extra about the way it interacts with different components will train us extra about the ones cycles too.In the case of cloud formation, it is important that we know how local weather exchange goes to impact the ambience and the clouds in it – every other space the place this find out about will turn out helpful at some point.For now, it solves probably the most mysteries of feldspar that had researchers confused. Earlier hypotheses had thought to be the results of the potassium atoms within the rock, and the defects in its crystal construction.”Researchers had been making an allowance for a number of concepts why feldspar is such an efficient nucleation seed,” says Diebold.The analysis has been printed within the Magazine of Bodily Chemistry Letters.
We Now Know Why This Not unusual Rock Is So Just right at Rising Clouds