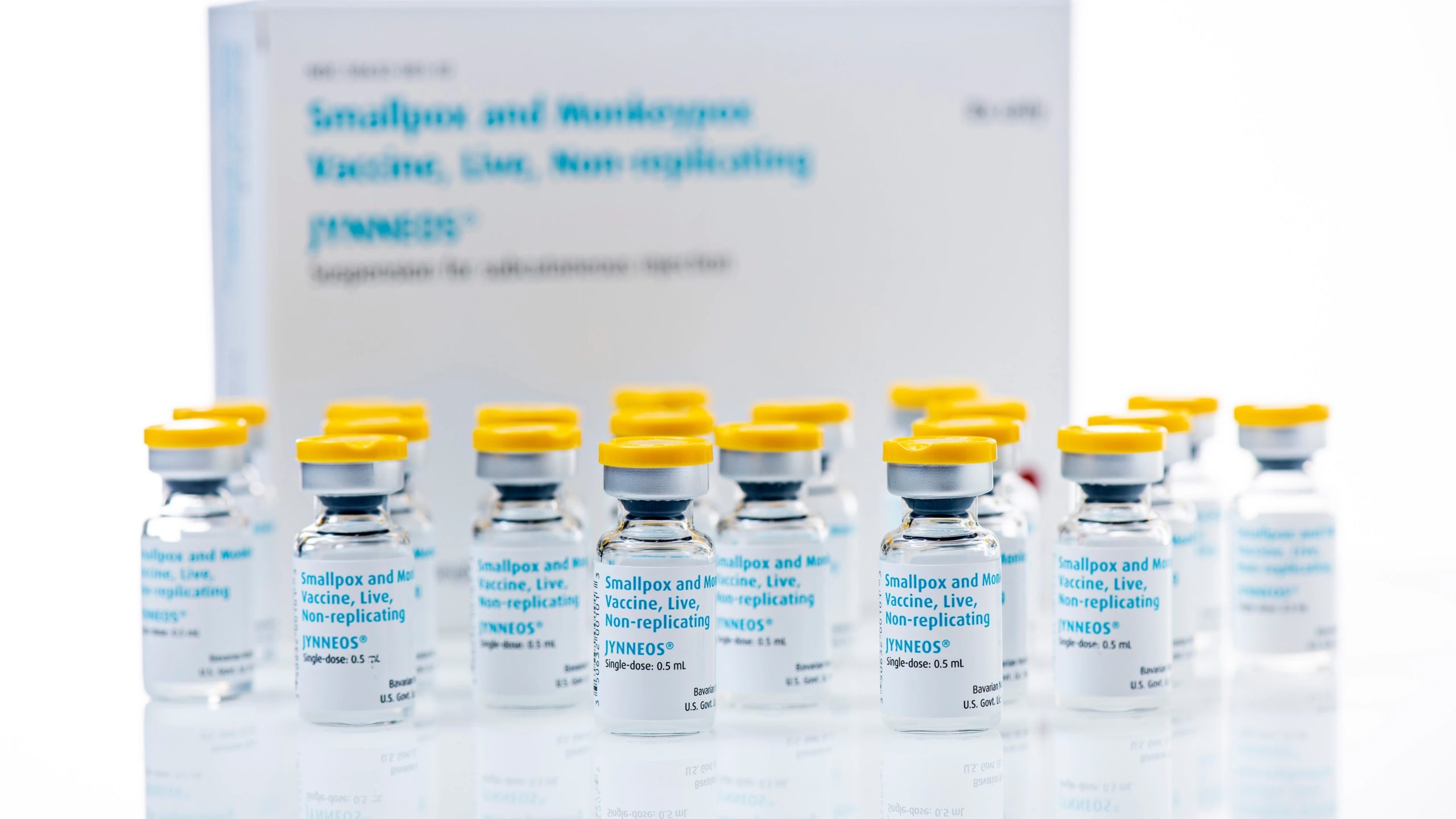
Bavarian Nordic’s MVA-BN vaccine, has grow to be the primary mpox vaccine to be added to the Global Well being Group (WHO) prequalification (PQ) listing, enabling its use globally.
The WHO has additionally advisable that unmarried doses be given in supply-constrained outbreak eventualities as an alternative of the standard two doses, and “off label” use for kids because the vaccine isn’t but licensed for under-18s.
WHO Prequalification (PQ) and Emergency Use List (EUL) are mechanisms used to judge high quality, protection and efficacy of clinical merchandise, equivalent to vaccines, diagnostics and drugs, and product suitability to be used in low- and middle-income nations.
“PQ is in line with the overview of complete set of high quality, protection and efficacy information on clinical merchandise, together with possibility control plan and programmatic suitability,” in step with a Friday media liberate from WHO.
“WHO’s evaluation for prequalification is in line with knowledge submitted via the producer, Bavarian Nordic, and overview via the Eu Medications Company, the regulatory company of file for this vaccine,” the WHO added.
A month in the past, WHO Director Basic Dr Tedros Adhanom Ghebreyesus declared mpox a public well being emergency of world worry (PHEIC) because the outbreak intensified within the Democratic Republic of the Congo (DRC) and neighbouring nations.
Because the international outbreak in 2022, over 120 nations have showed greater than 103 000 circumstances.
This 12 months, there were 25 237 suspected and showed circumstances and 723 deaths from other outbreaks in 14 African nations (8 September 2024). Morocco reported its first case on Thursday.
‘Off label’ for kids

“This primary prequalification of a vaccine towards mpox is the most important step in our combat towards the illness, each within the context of the present outbreaks in Africa, and in long term,” stated Tedros.
“We now want pressing scale up in procurement, donations and rollout to make sure equitable get entry to to vaccines the place they’re wanted maximum, along different public well being gear, to stop infections, prevent transmission and save lives.”
The MVA-BN vaccine (advertised as Jynneous and Imvamune) is run in other people over the age of 18 as a two-dose injection given 4 weeks aside. After prior chilly garage, the vaccine can also be saved at 2–8°C for as much as 8 weeks.
Despite the fact that, MVA-BN isn’t but registered to be used in youngsters, the WHO’s head of Analysis and Construction, Dr Ana-Maria Restrepo, instructed a contemporary media briefing that the DRC may use the vaccine “off label” for kids, and that there have been quite a lot of research – together with scientific research – that had established its efficacy in youngsters. The vast majority of mpox circumstances in DRC are in youngsters.
The WHO additionally famous that it may well be used “off-label” for pregnant and immuno-compromised other people “in outbreak settings the place the advantages of vaccination outweigh the possible dangers”.
WHO has additionally advisable single-dose use in supply-constrained outbreak eventualities. To be had information displays {that a} single-dose MVA-BN vaccine given earlier than publicity has an estimated 76% effectiveness while the two-dose time table gives an estimated 82% coverage.
Lend a hand for nationwide regulators
“The WHO prequalification of the MVA-BN vaccine will lend a hand boost up ongoing procurement of the mpox vaccines via governments and world businesses equivalent to Gavi and Unicef to lend a hand communities at the frontlines of the continued emergency in Africa and past,” stated Dr Yukiko Nakatani, WHO Assistant Director-Basic for Get right of entry to to Medications and Well being Merchandise.
“The verdict too can lend a hand nationwide regulatory government to fast-track approvals, in the end expanding get entry to to quality-assured mpox vaccine merchandise,” he added.
The WHO’s Strategic Advisory Crew of Professionals (SAGE) on Immunization reviewed all to be had proof and advisable using MVA-BN vaccine in mpox outbreaks for other people “at top possibility of publicity”.
Bavarian Nordic CEO Paul Chaplin stated that his corporate is “extremely inspired” via the PQ, “which is a testomony to the strengths of our vaccine and the standard of information now we have generated thru a large number of research, in addition to in actual existence”.
Corporate goals for 2 million doses in 2024
“Bavarian Nordic has just lately filed a submission to the Eu Medications Company to increase the approval to youngsters 12-17 years of age and could also be operating with companions, together with the Coalition for Epidemic Preparedness Inventions (CEPI) to judge the protection and efficacy of the vaccine in youngsters 2-12 years of age,” in step with an organization observation.
“Whilst we proceed to paintings with WHO and different regulatory our bodies to amplify the approval to incorporate youngsters, who’re critically impacted via the mpox outbreak, we’re happy that this approval will lend a hand boost up get entry to to our vaccine for communities throughout all of the African continent and we applaud the WHO for his or her swift overview and motion to make this occur,” added Chaplin.
Bavarian Nordic has undertaken to focal point its manufacturing efforts on MVA-BN, which is able to permit it to provide two million doses via the tip of the 12 months, and probably 13 million via the tip of 2025, the corporate reported on Thursday.
Dr Rogerio Gaspar, WHO Director for Law and Prequalification, stated the worldwide frame used to be “progressing with prequalification and emergency use checklist procedures with producers of 2 different mpox vaccines: LC-16 and ACAM2000. Now we have additionally won six expressions of hobby for mpox diagnostic merchandise for emergency use checklist to this point”.
The LC-16 vaccine, produced via Japan’s KM Biologics, is authorized to be used in youngsters.
Symbol Credit: US Centres for Illness Regulate .
Battle the infodemic in well being knowledge and reinforce well being coverage reporting from the worldwide South. Our rising community of reporters in Africa, Asia, Geneva and New York attach the dots between regional realities and the massive international debates, with evidence-based, open get entry to information and research. To make a non-public or organisational contribution click on right here on PayPal.